Diffuse lung disease of infancy: a pattern-based, algorithmic approach to histological diagnosis
- PMID: 25477529
- PMCID: PMC4316934
- DOI: 10.1136/jclinpath-2014-202685
Diffuse lung disease of infancy: a pattern-based, algorithmic approach to histological diagnosis
Abstract
Diffuse lung disease (DLD) of infancy has multiple aetiologies and the spectrum of disease is substantially different from that seen in older children and adults. In many cases, a specific diagnosis renders a dire prognosis for the infant, with profound management implications. Two recently published series of DLD of infancy, collated from the archives of specialist centres, indicate that the majority of their cases were referred, implying that the majority of biopsies taken for DLD of infancy are first received by less experienced pathologists. The current literature describing DLD of infancy takes a predominantly aetiological approach to classification. We present an algorithmic, histological, pattern-based approach to diagnosis of DLD of infancy, which, with the aid of appropriate multidisciplinary input, including clinical and radiological expertise and ancillary diagnostic studies, may lead to an accurate and useful interim report, with timely exclusion of inappropriate diagnoses. Subsequent referral to a specialist centre for confirmatory diagnosis will be dependent on the individual case and the decision of the multidisciplinary team.
Keywords: HISTOPATHOLOGY; LUNG; PAEDIATRIC PATHOLOGY.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures

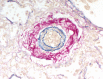

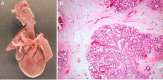


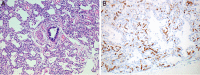
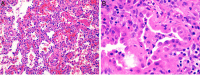



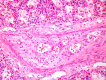
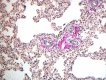

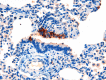
References
-
- Langston C, Dishop MK. Diffuse lung disease in infancy: a proposed classification applied to 259 diagnostic biopsies. Pediatr Dev Pathol 2009;12:421–37. - PubMed
-
- Rice A, Tran-Dang MA, Bush A, et al. Diffuse lung disease in infancy and childhood: expanding the chILD classification. Histopathology 2013;63:743–55. - PubMed
-
- Sebire NJ, Malone M, Ashworth M, et al. Diagnostic pediatric surgical pathology. New York: Churchill Livingstone Elsevier, 2010; Chapter 12, Respiratory Pathology.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
