Molecular pathways: cbl proteins in tumorigenesis and antitumor immunity-opportunities for cancer treatment
- PMID: 25477533
- PMCID: PMC4401614
- DOI: 10.1158/1078-0432.CCR-13-2490
Molecular pathways: cbl proteins in tumorigenesis and antitumor immunity-opportunities for cancer treatment
Abstract
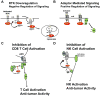
The Cbl proteins are a family of ubiquitin ligases (E3s) that regulate signaling through many tyrosine kinase-dependent pathways. A predominant function is to negatively regulate receptor tyrosine kinase (RTK) signaling by ubiquitination of active RTKs, targeting them for trafficking to the lysosome for degradation. Also, Cbl-mediated ubiquitination can regulate signaling protein function by altered cellular localization of proteins without degradation. In addition to their role as E3s, Cbl proteins play a positive role in signaling by acting as adaptor proteins that can recruit signaling molecules to the active RTKs. Cbl-b, a second family member, negatively regulates the costimulatory pathway of CD8 T cells and also negatively regulates natural killer cell function. The different functions of Cbl proteins and their roles both in the development of cancer and the regulation of immune responses provide multiple therapeutic opportunities. Mutations in Cbl that inactivate the negative E3 function while maintaining the positive adaptor function have been described in approximately 5% of myeloid neoplasms. An improved understanding of how the signaling pathways [e.g., Fms-like tyrosine kinase 3 (Flt3), PI3K, and signal transducer and activator of transcription (Stat)] are dysregulated by these mutations in Cbl has helped to identify potential targets for therapy of myeloid neoplasms. Conversely, the loss of Cbl-b leads to increased adaptive and innate antitumor immunity, suggesting that inhibiting Cbl-b may be a means to increase antitumor immunity across a wide variety of tumors. Thus, targeting the pathways regulated by Cbl proteins may provide attractive opportunities for treating cancer.
©2014 American Association for Cancer Research.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures
References
-
- Tsygankov A, editor. Cbl proteins. New York: Nova Science Publishers; 2008.
-
- Nau MM, Lipkowitz S. Welcome to the family: Cbl-family gene organization, overview of structure and functions of Cbl-related proteins in various taxonomical groups. In: Tsygankov AY, editor. Cbl proteins. New York: Nova Science Publishers; 2008. pp. 3–25.
-
- Schmidt MH, Dikic I. The Cbl interactome and its functions. Nat Rev Mol Cell Biol. 2005;6:907–19. - PubMed
-
- Rao N, Dodge I, Band H. The Cbl family of ubiquitin ligases: critical negative regulators of tyrosine kinase signaling in the immune system. J Leukoc Biol. 2002;71:753–63. - PubMed
-
- Thien CB, Langdon WY. Cbl: many adaptations to regulate protein tyrosine kinases. Nat Rev Mol Cell Biol. 2001;2:294–307. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

