Differentially expressed microRNAs in maternal plasma for the noninvasive prenatal diagnosis of Down syndrome (trisomy 21)
- PMID: 25478570
- PMCID: PMC4244954
- DOI: 10.1155/2014/402475
Differentially expressed microRNAs in maternal plasma for the noninvasive prenatal diagnosis of Down syndrome (trisomy 21)
Abstract
Objectives: Most developmental processes are under the control of small regulatory RNAs called microRNAs (miRNAs). We hypothesize that different fetal developmental processes might be reflected by extracellular miRNAs in maternal plasma and may be utilized as biomarkers for the noninvasive prenatal diagnosis of chromosomal aneuploidies. In this proof-of-concept study, we report on the identification of extracellular miRNAs in maternal plasma of Down syndrome (DS) pregnancies.
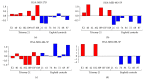
Methods: Using high-throughput quantitative PCR (HT-qPCR), 1043 miRNAs were investigated in maternal plasma via comparison of seven DS pregnancies with age and fetal sex matched controls.
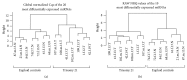
Results: Six hundred and ninety-five miRNAs were identified. Thirty-six significantly differentially expressed mature miRNAs were identified as potential biomarkers. Hierarchical cluster analysis of these miRNAs resulted in the clear discrimination of DS from euploid pregnancies. Gene targets of the differentially expressed miRNAs were enriched in signaling pathways such as mucin type-O-glycans, ECM-receptor interactions, TGF-beta, and endocytosis, which have been previously associated with DS.
Conclusions: miRNAs are promising and stable biomarkers for a broad range of diseases and may allow a reliable, cost-efficient diagnostic tool for the noninvasive prenatal diagnosis of DS.
Figures

References
-
- Mégarbané A., Ravel A., Mircher C., Sturtz F., Grattau Y., Rethoré M.-O., Delabar J.-M., Mobley W. C. The 50th anniversary of the discovery of trisomy 21: the past, present, and future of research and treatment of Down syndrome. Genetics in Medicine. 2009;11(9):611–616. doi: 10.1097/GIM.0b013e3181b2e34c. - DOI - PubMed
-
- Cohen W. I. Health care guidelines for individuals with Down Syndrome. Down Syndrome Quarterly. 1999;4(3):1–16.
-
- Lo Y. M. D. Fetal DNA in maternal plasma: biology and diagnostic applications. Clinical Chemistry. 2000;46(12):1903–1906. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

