Impact of methionine oxidation on calmodulin structural dynamics
- PMID: 25478640
- PMCID: PMC4312012
- DOI: 10.1016/j.bbrc.2014.11.091
Impact of methionine oxidation on calmodulin structural dynamics
Abstract
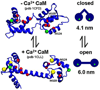
We have used electron paramagnetic resonance (EPR) to examine the structural impact of oxidizing specific methionine (M) side chains in calmodulin (CaM). It has been shown that oxidation of either M109 or M124 in CaM diminishes CaM regulation of the muscle calcium release channel, the ryanodine receptor (RyR), and that mutation of M to Q (glutamine) in either case produces functional effects identical to those of oxidation. Here we have used site-directed spin labeling and double electron-electron resonance (DEER), a pulsed EPR technique that measures distances between spin labels, to characterize the structural changes resulting from these mutations. Spin labels were attached to a pair of introduced cysteine residues, one in the C-lobe (T117C) and one in the N-lobe (T34C) of CaM, and DEER was used to determine the distribution of interspin distances. Ca binding induced a large increase in the mean distance, in concert with previous X-ray crystallography and NMR data, showing a closed structure in the absence of Ca and an open structure in the presence of Ca. DEER revealed additional information about CaM's structural heterogeneity in solution: in both the presence and absence of Ca, CaM populates both structural states, one with probes separated by ∼4nm (closed) and another at ∼6nm (open). Ca shifts the structural equilibrium constant toward the open state by a factor of 13. DEER reveals the distribution of interprobe distances, showing that each of these states is itself partially disordered, with the width of each population ranging from 1 to 3nm. Both mutations (M109Q and M124Q) decrease the effect of Ca on the structure of CaM, primarily by decreasing the closed-to-open equilibrium constant in the presence of Ca. We propose that Met oxidation alters CaM's functional interaction with its target proteins by perturbing this Ca-dependent structural shift.
Keywords: Aging; DEER; Muscle; Oxidative stress; Pulsed EPR; Ryanodine receptor.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures

References
-
- Stadtman ER, Berlett BS. Reactive oxygen-mediated protein oxidation in aging and disease. Chem Res Toxicol. 1997;10:485–494. - PubMed
-
- Baraibar MA, Gueugneau M, Duguez S, Butler-Browne G, Bechet D, Friguet B. Expression and modification proteomics during skeletal muscle ageing. Biogerontology. 2013;14:339–352. - PubMed
-
- Tidball JG, Wehling-Henricks M. The role of free radicals in the pathophysiology of muscular dystrophy. J Appl Physiol. 2007;102:1677–1686. - PubMed
-
- Maack C, Kartes T, Kilter H, Schafers HJ, Nickenig G, Bohm M, Laufs U. Oxygen free radical release in human failing myocardium is associated with increased activity of rac1-GTPase and represents a target for statin treatment. Circulation. 2003;108:1567–1574. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

