Impact of prior chemotherapy use on the efficacy of everolimus in patients with advanced pancreatic neuroendocrine tumors: a subgroup analysis of the phase III RADIANT-3 trial
- PMID: 25479584
- PMCID: PMC4327560
- DOI: 10.1097/MPA.0000000000000262
Impact of prior chemotherapy use on the efficacy of everolimus in patients with advanced pancreatic neuroendocrine tumors: a subgroup analysis of the phase III RADIANT-3 trial
Abstract
Objective: The aim of this study was to evaluate efficacy and safety of everolimus in patients with pancreatic neuroendocrine tumors (pNET) by prior chemotherapy use in the RAD001 in Advanced Neuroendocrine Tumors, Third Trial (RADIANT-3).
Methods: Patients with advanced, progressive, low- or intermediate-grade pNET were prospectively stratified by prior chemotherapy use and World Health Organization performance status and were randomly assigned (1:1) to everolimus 10 mg/d (n = 207) or placebo (n = 203).
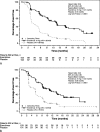
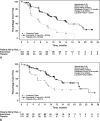
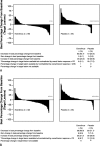
Results: Of the 410 patients, 204 (50%) were naive to chemotherapy (chemonaive). Baseline characteristics were similar for patients with or without prior chemotherapy. Everolimus significantly prolonged median progression-free survival regardless of prior chemotherapy use (prior chemotherapy: 11.0 vs 3.2 months; hazard ratio, 0.34; 95% confidence interval, 0.25-0.48; P < 0.0001) (chemonaive: 11.4 vs 5.4 months; hazard ratio, 0.42; 95% confidence interval, 0.29-0.60; P < 0.0001). Stable disease was the best overall response in 73% of everolimus-treated patients (151/207). The most common drug-related adverse events included stomatitis (60%-69%), rash (47%-50%), and diarrhea (34%).
Conclusions: As more treatment options become available, it is important to consider the goals of treatment and to identify patients who would potentially benefit from a specific therapy. Findings from this planned subgroup analysis suggest the potential for first-line use of everolimus in patients with advanced pNET.
Trial registration: ClinicalTrials.gov NCT00510068.
Figures
References
-
- Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008; 26: 3063– 3072. - PubMed
-
- Phan AT, Yao JC. Neuroendocrine tumors: novel approaches in the age of targeted therapy. Oncology. 2008; 22: 1617– 1623; discussion 1623–1624, 1629. - PubMed
-
- Yao J, Phan AT. Optimising therapeutic options for patients with advanced pancreatic neuroendocrine tumours. Eur Oncol Haematol. 2012; 8: 217– 223.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

