Telomerase inhibition effectively targets mouse and human AML stem cells and delays relapse following chemotherapy
- PMID: 25479751
- PMCID: PMC4317339
- DOI: 10.1016/j.stem.2014.11.010
Telomerase inhibition effectively targets mouse and human AML stem cells and delays relapse following chemotherapy
Abstract
Acute myeloid leukemia (AML) is an aggressive and lethal blood cancer maintained by rare populations of leukemia stem cells (LSCs). Selective targeting of LSCs is a promising approach for treating AML and preventing relapse following chemotherapy, and developing such therapeutic modalities is a key priority. Here, we show that targeting telomerase activity eradicates AML LSCs. Genetic deletion of the telomerase subunit Terc in a retroviral mouse AML model induces cell-cycle arrest and apoptosis of LSCs, and depletion of telomerase-deficient LSCs is partially rescued by p53 knockdown. Murine Terc(-/-) LSCs express a specific gene expression signature that can be identified in human AML patient cohorts and is positively correlated with patient survival following chemotherapy. In xenografts of primary human AML, genetic or pharmacological inhibition of telomerase targets LSCs, impairs leukemia progression, and delays relapse following chemotherapy. Altogether, these results establish telomerase inhibition as an effective strategy for eliminating AML LSCs.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures


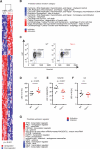


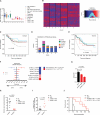
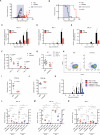
Comment in
-
Pushing the limits: defeating leukemia stem cells by depleting telomerase.Cell Stem Cell. 2014 Dec 4;15(6):673-5. doi: 10.1016/j.stem.2014.11.014. Cell Stem Cell. 2014. PMID: 25479742 Free PMC article.
References
-
- Allsopp RC, Morin GB, DePinho R, Harley CB, Weissman IL. Telomerase is required to slow telomere shortening and extend replicative lifespan of HSCs during serial transplantation. Blood. 2003;102:517–520. - PubMed
-
- Bagger FO, Rapin N, Theilgaard-Monch K, Kaczkowski B, Jendholm J, Winther O, Porse B. HemaExplorer: a Web server for easy and fast visualization of gene expression in normal and malignant hematopoiesis. Blood. 2012;119:6394–6395. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

