Detection of distinct glycosylation patterns on human γ-glutamyl transpeptidase 1 using antibody-lectin sandwich array (ALSA) technology
- PMID: 25479762
- PMCID: PMC4297448
- DOI: 10.1186/s12896-014-0101-0
Detection of distinct glycosylation patterns on human γ-glutamyl transpeptidase 1 using antibody-lectin sandwich array (ALSA) technology
Abstract
Background: γ-Glutamyl transpeptidase 1 (GGT1) is an N-glycosylated membrane protein that catabolizes extracellular glutathione and other γ-glutamyl-containing substrates. In a variety of disease states, including tumor formation, the enzyme is shed from the surface of the cell and can be detected in serum. The structures of the N-glycans on human GGT1 (hGGT1) have been shown to be tissue-specific. Tumor-specific changes in the glycans have also been observed, suggesting that the N-glycans on hGGT1 would be an important biomarker for detecting tumors and monitoring their progression during treatment. However, the large quantities of purified protein required to fully characterize the carbohydrate content poses a significant challenge for biomarker development. Herein, we investigated a new antibody-lectin sandwich array (ALSA) platform to determine whether this microanalytical technique could be applied to the characterization of N-glycan content of hGGT1 in complex biological samples.
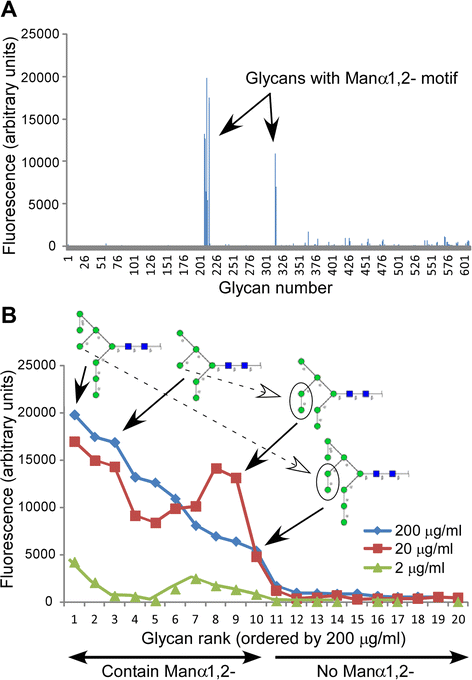
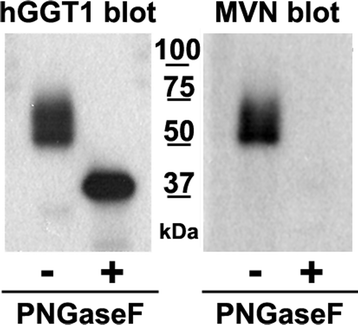
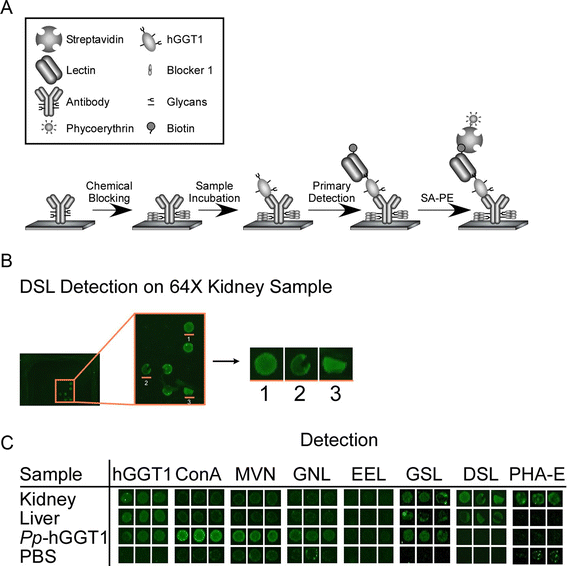
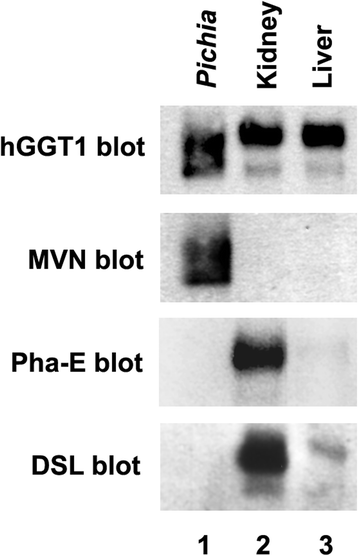
Results: Our data show that hGGT1 can be isolated from detergent extracted membrane proteins by binding to the ALSA platform. Probing hGGT1 with lectins enables characterization of the N-glycans. We probed hGGT1 from normal human liver tissue, normal human kidney tissue, and hGGT1 expressed in the yeast Pichia pastoris. The lectin binding patterns obtained with the ALSA platform are consistent with the hGGT1 N-glycan composition obtained from previous large-scale hGGT1 N-glycan characterizations from these sources. We also validate the implementation of the Microcystis aeruginosa lectin, microvirin, in this platform and provide refined evidence for its efficacy in specifically recognizing high-mannose-type N-glycans, a class of carbohydrate modification that is distinctive of hGGT1 expressed by many tumors.
Conclusion: Using this microanalytical approach, we provide proof-of-concept for the implementation of ALSA in conducting high-throughput studies aimed at investigating disease-related changes in the glycosylation patterns on hGGT1 with the goal of enhancing clinical diagnoses and targeted treatment regimens.
Figures




References
-
- Lieberman MW, Wiseman AL, Shi ZZ, Carter BZ, Barrios R, Ou CN, Chevez-Barrios P, Wang Y, Habib GM, Goodman JC, Huang SL, Lebovitz RM, Matzuk MM. Growth retardation and cysteine deficiency in gamma-glutamyl transpeptidase-deficient mice. Proc Natl Acad Sci U S A. 1996;93(15):7923–7926. doi: 10.1073/pnas.93.15.7923. - DOI - PMC - PubMed
-
- Hanigan MH, Gallagher BC, Taylor PT, Jr, Large MK. Inhibition of gamma-glutamyl transpeptidase activity by acivicin in vivo protects the kidney from cisplatin-induced toxicity. Cancer Res. 1994;54(22):5925–5929. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

