Cytokine profiles in pregnant gilts experimentally infected with porcine reproductive and respiratory syndrome virus and relationships with viral load and fetal outcome
- PMID: 25479904
- PMCID: PMC4333882
- DOI: 10.1186/s13567-014-0113-8
Cytokine profiles in pregnant gilts experimentally infected with porcine reproductive and respiratory syndrome virus and relationships with viral load and fetal outcome
Abstract
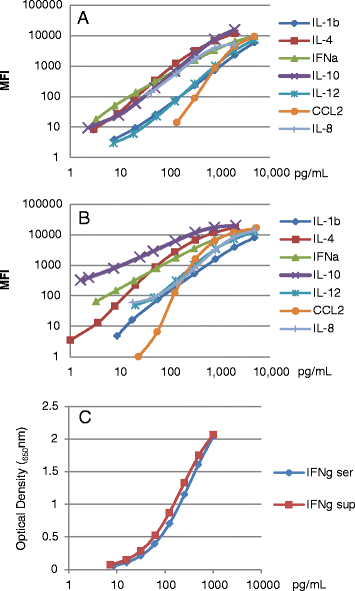
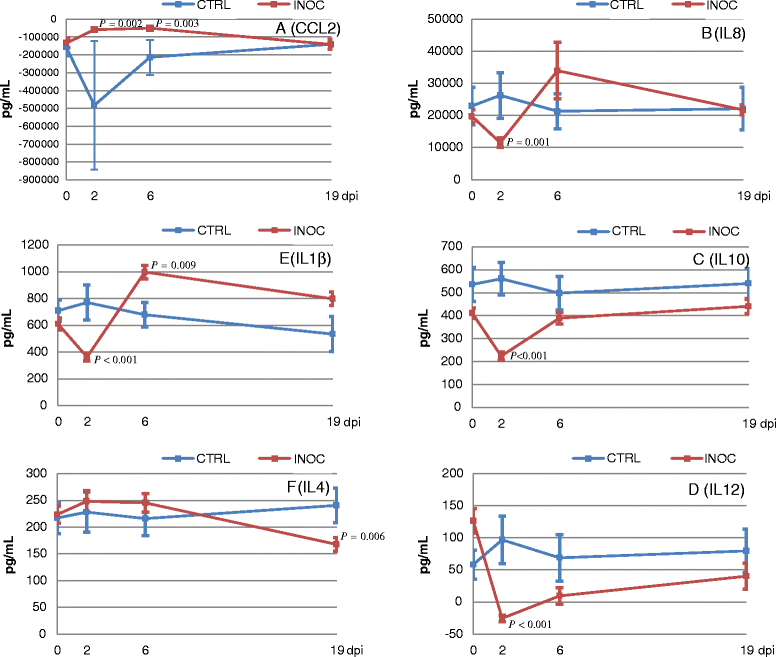
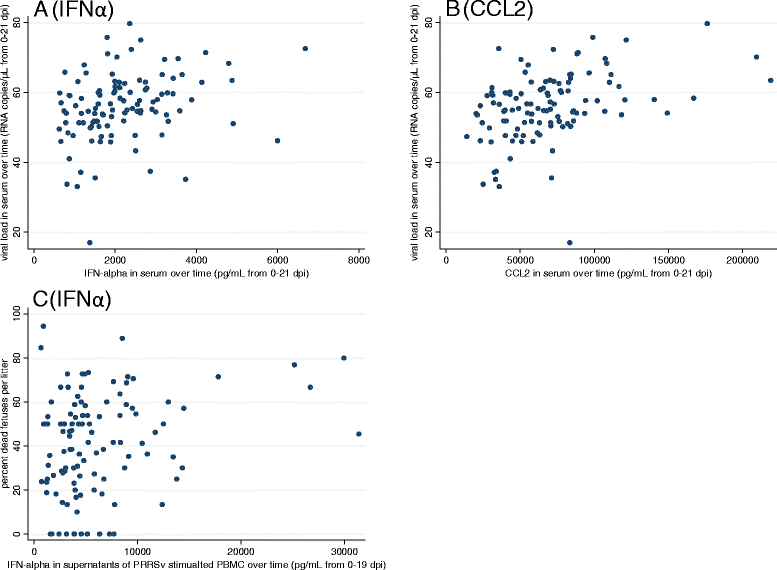
In spite of extensive research, immunologic control mechanisms against Porcine Reproductive and Respiratory Syndrome virus (PRRSv) remain poorly understood. Cytokine responses have been exhaustively studied in nursery pigs and show contradictory results. Since no detailed reports on cytokine responses to PRRSv in pregnant females exist, the objectives of this study were to compare host cytokine responses between PRRSv-infected and non-infected pregnant gilts, and to investigate relationships between cytokine levels in infected gilts and viral load or fetal mortality rate. Serum samples and supernatants of peripheral blood mononuclear cells (PBMC) either stimulated with PRRSv or phorbol myristate acetate/Ionomycin (PMA/Iono) were analyzed for cytokines/chemokines: interleukins (IL) 1-beta (IL1β), IL4, IL8, IL10, IL12, chemokine ligand 2 (CCL2), interferon alpha (IFNα) and interferon gamma (IFNγ). Three cytokines (IFNα, CCL2, IFNγ) in gilt serum differed significantly in inoculated versus control gilts over time. In supernatants of PRRSv stimulated PBMC from PRRSv-infected gilts, levels of IFNα were significantly decreased, while IL8 secretion was significantly increased. PRRSv infection altered the secretion of all measured cytokines, with the exception of IFNα, from PBMC after mitogen stimulation, indicating a possible immunomodulatory effect of PRRSv. IFNα, CCL2, and IFNγ in serum, and IFNγ in supernatants of PMA/Iono stimulated PBMC were significantly associated with viral load in tissues, serum or both. However, only IFNα in supernatants of PRRSv stimulated PBMC was significantly associated with fetal mortality rate. We conclude that of the eight cytokines tested in this study IFNα was the best indicator of viral load and severity of reproductive PRRSv infection.
Figures


Similar articles
-
Pathogenicity of three type 2 porcine reproductive and respiratory syndrome virus strains in experimentally inoculated pregnant gilts.Virus Res. 2015 May 4;203:24-35. doi: 10.1016/j.virusres.2015.03.005. Epub 2015 Mar 18. Virus Res. 2015. PMID: 25796212
-
Changes in leukocyte subsets of pregnant gilts experimentally infected with porcine reproductive and respiratory syndrome virus and relationships with viral load and fetal outcome.Vet Res. 2014 Dec 14;45(1):128. doi: 10.1186/s13567-014-0128-1. Vet Res. 2014. PMID: 25497114 Free PMC article.
-
Maternal and fetal predictors of fetal viral load and death in third trimester, type 2 porcine reproductive and respiratory syndrome virus infected pregnant gilts.Vet Res. 2015 Sep 25;46:107. doi: 10.1186/s13567-015-0251-7. Vet Res. 2015. PMID: 26407558 Free PMC article.
-
Adjuvants for porcine reproductive and respiratory syndrome virus vaccines.Vet Immunol Immunopathol. 2009 May 15;129(1-2):1-13. doi: 10.1016/j.vetimm.2008.12.018. Epub 2008 Dec 11. Vet Immunol Immunopathol. 2009. PMID: 19157569 Review.
-
Novel insights into host responses and reproductive pathophysiology of porcine reproductive and respiratory syndrome caused by PRRSV-2.Vet Microbiol. 2017 Sep;209:114-123. doi: 10.1016/j.vetmic.2017.02.019. Epub 2017 Mar 2. Vet Microbiol. 2017. PMID: 28292546 Review.
Cited by
-
Maternal and fetal thyroid dysfunction following porcine reproductive and respiratory syndrome virus2 infection.Vet Res. 2020 Mar 30;51(1):47. doi: 10.1186/s13567-020-00772-2. Vet Res. 2020. PMID: 32228691 Free PMC article.
-
Differences in Whole Blood Gene Expression Associated with Infection Time-Course and Extent of Fetal Mortality in a Reproductive Model of Type 2 Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) Infection.PLoS One. 2016 Apr 19;11(4):e0153615. doi: 10.1371/journal.pone.0153615. eCollection 2016. PLoS One. 2016. PMID: 27093427 Free PMC article.
-
Expression of T-Bet, Eomesodermin, and GATA-3 Correlates With Distinct Phenotypes and Functional Properties in Porcine γδ T Cells.Front Immunol. 2019 Mar 11;10:396. doi: 10.3389/fimmu.2019.00396. eCollection 2019. Front Immunol. 2019. PMID: 30915070 Free PMC article.
-
Thyroid hormone suppression in feeder pigs following polymicrobial or porcine reproductive and respiratory syndrome virus-2 challenge.J Anim Sci. 2021 Nov 1;99(11):skab325. doi: 10.1093/jas/skab325. J Anim Sci. 2021. PMID: 34734242 Free PMC article.
-
Comparison of clinical and immunological findings in gnotobiotic piglets infected with Escherichia coli O104:H4 outbreak strain and EHEC O157:H7.Gut Pathog. 2017 May 25;9:30. doi: 10.1186/s13099-017-0179-8. eCollection 2017. Gut Pathog. 2017. PMID: 28559930 Free PMC article.
References
-
- Murphy KP. Janeway’s Immunobiology. New York, USA: Garland Science, Taylor & Francis Group, LLC; 2012.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

