Environment drives selection and function of enhancers controlling tissue-specific macrophage identities
- PMID: 25480297
- PMCID: PMC4364385
- DOI: 10.1016/j.cell.2014.11.023
Environment drives selection and function of enhancers controlling tissue-specific macrophage identities
Abstract
Macrophages reside in essentially all tissues of the body and play key roles in innate and adaptive immune responses. Distinct populations of tissue macrophages also acquire context-specific functions that are important for normal tissue homeostasis. To investigate mechanisms responsible for tissue-specific functions, we analyzed the transcriptomes and enhancer landscapes of brain microglia and resident macrophages of the peritoneal cavity. In addition, we exploited natural genetic variation as a genome-wide "mutagenesis" strategy to identify DNA recognition motifs for transcription factors that promote common or subset-specific binding of the macrophage lineage-determining factor PU.1. We find that distinct tissue environments drive divergent programs of gene expression by differentially activating a common enhancer repertoire and by inducing the expression of divergent secondary transcription factors that collaborate with PU.1 to establish tissue-specific enhancers. These findings provide insights into molecular mechanisms by which tissue environment influences macrophage phenotypes that are likely to be broadly applicable to other cell types.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




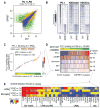

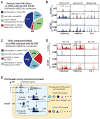
Comment in
-
Environmentally-defined enhancer populations regulate diversity of tissue-resident macrophages.Trends Immunol. 2015 Feb;36(2):61-2. doi: 10.1016/j.it.2014.12.002. Epub 2015 Jan 7. Trends Immunol. 2015. PMID: 25575465 Free PMC article.
References
-
- Andrews NC. The NF-E2 transcription factor. The international journal of biochemistry & cell biology. 1998;30:429–432. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- P01 HL088093/HL/NHLBI NIH HHS/United States
- WT101853MA/WT_/Wellcome Trust/United Kingdom
- P30 DK063491/DK/NIDDK NIH HHS/United States
- GM085764/GM/NIGMS NIH HHS/United States
- P30 CA023100/CA/NCI NIH HHS/United States
- DK091183/DK/NIDDK NIH HHS/United States
- DK063491/DK/NIDDK NIH HHS/United States
- 1K99HL12348/HL/NHLBI NIH HHS/United States
- T32 GM007198/GM/NIGMS NIH HHS/United States
- P01 DK074868/DK/NIDDK NIH HHS/United States
- R01 CA173903/CA/NCI NIH HHS/United States
- K99 HL123485/HL/NHLBI NIH HHS/United States
- CA17390/CA/NCI NIH HHS/United States
- R01 CA052599/CA/NCI NIH HHS/United States
- R01 DK091183/DK/NIDDK NIH HHS/United States
- P50 GM085764/GM/NIGMS NIH HHS/United States
- 101853/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

