A systems biology approach to iron metabolism
- PMID: 25480643
- PMCID: PMC4464783
- DOI: 10.1007/978-1-4939-2095-2_10
A systems biology approach to iron metabolism
Abstract
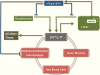
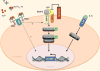
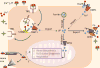
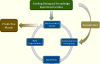
Iron is critical to the survival of almost all living organisms. However, inappropriately low or high levels of iron are detrimental and contribute to a wide range of diseases. Recent advances in the study of iron metabolism have revealed multiple intricate pathways that are essential to the maintenance of iron homeostasis. Further, iron regulation involves processes at several scales, ranging from the subcellular to the organismal. This complexity makes a systems biology approach crucial, with its enabling technology of computational models based on a mathematical description of regulatory systems. Systems biology may represent a new strategy for understanding imbalances in iron metabolism and their underlying causes.
Figures

References
-
- Josephs HW. Absorption of iron as a problem in human physiology; a critical review. Blood. 1958;13(1):1–54. - PubMed
-
- Laufberger V. Sur la cristallisation de la ferritine. Soc Chim Biol. 1937;19:1575–1582.
-
- Feder JN, et al. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet. 1996;13(4):399–408. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

