Randomised placebo-controlled trials of individualised homeopathic treatment: systematic review and meta-analysis
- PMID: 25480654
- PMCID: PMC4326322
- DOI: 10.1186/2046-4053-3-142
Randomised placebo-controlled trials of individualised homeopathic treatment: systematic review and meta-analysis
Abstract
Background: A rigorous and focused systematic review and meta-analysis of randomised controlled trials (RCTs) of individualised homeopathic treatment has not previously been undertaken. We tested the hypothesis that the outcome of an individualised homeopathic treatment approach using homeopathic medicines is distinguishable from that of placebos.
Methods: The review's methods, including literature search strategy, data extraction, assessment of risk of bias and statistical analysis, were strictly protocol-based. Judgment in seven assessment domains enabled a trial's risk of bias to be designated as low, unclear or high. A trial was judged to comprise 'reliable evidence' if its risk of bias was low or was unclear in one specified domain. 'Effect size' was reported as odds ratio (OR), with arithmetic transformation for continuous data carried out as required; OR > 1 signified an effect favouring homeopathy.
Results: Thirty-two eligible RCTs studied 24 different medical conditions in total. Twelve trials were classed 'uncertain risk of bias', three of which displayed relatively minor uncertainty and were designated reliable evidence; 20 trials were classed 'high risk of bias'. Twenty-two trials had extractable data and were subjected to meta-analysis; OR = 1.53 (95% confidence interval (CI) 1.22 to 1.91). For the three trials with reliable evidence, sensitivity analysis revealed OR = 1.98 (95% CI 1.16 to 3.38).
Conclusions: Medicines prescribed in individualised homeopathy may have small, specific treatment effects. Findings are consistent with sub-group data available in a previous 'global' systematic review. The low or unclear overall quality of the evidence prompts caution in interpreting the findings. New high-quality RCT research is necessary to enable more decisive interpretation.
Figures

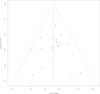

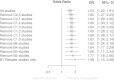
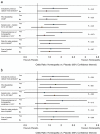
Similar articles
-
Homeopathic medicinal products for preventing and treating acute respiratory tract infections in children.Cochrane Database Syst Rev. 2018 Sep 9;9(9):CD005974. doi: 10.1002/14651858.CD005974.pub5. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2022 Dec 13;12:CD005974. doi: 10.1002/14651858.CD005974.pub6. PMID: 30196554 Free PMC article. Updated.
-
Homeopathic medicinal products for preventing and treating acute respiratory tract infections in children.Cochrane Database Syst Rev. 2018 Apr 9;4(4):CD005974. doi: 10.1002/14651858.CD005974.pub4. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2018 Sep 09;9:CD005974. doi: 10.1002/14651858.CD005974.pub5. PMID: 29630715 Free PMC article. Updated.
-
Randomised, double-blind, placebo-controlled trials of non-individualised homeopathic treatment: systematic review and meta-analysis.Syst Rev. 2017 Mar 24;6(1):63. doi: 10.1186/s13643-017-0445-3. Syst Rev. 2017. PMID: 28340607 Free PMC article.
-
Homeopathy for treatment of irritable bowel syndrome.Cochrane Database Syst Rev. 2013 Nov 13;(11):CD009710. doi: 10.1002/14651858.CD009710.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2019 Sep 04;9:CD009710. doi: 10.1002/14651858.CD009710.pub3. PMID: 24222383 Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
Cited by
-
A Randomized Three-Arm Double-Blind Placebo-Controlled Study of Homeopathic Treatment of Children and Youth with Attention-Deficit/Hyperactivity Disorder.J Integr Complement Med. 2024 Mar;30(3):279-287. doi: 10.1089/jicm.2023.0043. Epub 2023 Sep 6. J Integr Complement Med. 2024. PMID: 37672605 Free PMC article. Clinical Trial.
-
Homeopathic medicinal products for preventing and treating acute respiratory tract infections in children.Cochrane Database Syst Rev. 2018 Sep 9;9(9):CD005974. doi: 10.1002/14651858.CD005974.pub5. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2022 Dec 13;12:CD005974. doi: 10.1002/14651858.CD005974.pub6. PMID: 30196554 Free PMC article. Updated.
-
Pre-admission interventions to improve outcome after elective surgery-protocol for a systematic review.Syst Rev. 2016 May 23;5:88. doi: 10.1186/s13643-016-0266-9. Syst Rev. 2016. PMID: 27216584 Free PMC article.
-
The current state of the quality of homeopathic clinical research.Complement Ther Med. 2025 Mar;88:103108. doi: 10.1016/j.ctim.2024.103108. Epub 2024 Nov 16. Complement Ther Med. 2025. PMID: 39551363 Free PMC article. Review.
-
Homeopathic medicinal products for preventing and treating acute respiratory tract infections in children.Cochrane Database Syst Rev. 2018 Apr 9;4(4):CD005974. doi: 10.1002/14651858.CD005974.pub4. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2018 Sep 09;9:CD005974. doi: 10.1002/14651858.CD005974.pub5. PMID: 29630715 Free PMC article. Updated.
References
-
- Vithoulkas G: Another point of view for the homeopathic trials and meta-analyses. [http://www.vithoulkas.com/en/research/articles/2247.html]
-
- Sense About Science: Homeopathy. .] [http://www.senseaboutscience.org/data/files/resources/54/Homeopathy.pdf
-
- Boissel JP, Cucherat M, Haugh M, Gauthier E. Homoeopathic Medicine Research Group, Report of the Commission of the European Communities, Directorate-General XII – Science, Research and Development, Directorate E – RTD Actions. Brussels, Belgium: Life Sciences and Technologies – Medical Research; 1996. Critical literature review on the effectiveness of homoeopathy: overview of data from homoeopathic medicine trials.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

