The TRPM8 protein is a testosterone receptor: I. Biochemical evidence for direct TRPM8-testosterone interactions
- PMID: 25480783
- PMCID: PMC4317017
- DOI: 10.1074/jbc.M114.610824
The TRPM8 protein is a testosterone receptor: I. Biochemical evidence for direct TRPM8-testosterone interactions
Abstract
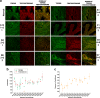
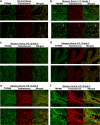
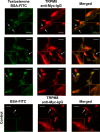
The transient receptor potential ion channel of the melastatin subfamily, TRPM8, is a major cold receptor in the peripheral nervous system. Along with the sensory neurons, the TRPM8 protein is highly expressed in the prostate epithelial cells, and this expression is regulated by androgens. Here we investigated the expression and intracellular localization of the TRPM8 channel in relationship to androgens. We performed experiments using human prostate tissues obtained from healthy individuals and patients with prostate cancer at various stages of the disease as well as in cultured cells. Using an immunohistochemistry approach, we detected an intensive colocalization pattern of the TRPM8 protein with endogenous androgens in all tissues tested, suggesting possible interactions. Co-immunoprecipitation experiments performed using cultured prostate epithelial cells, prostate cancer cells, and HEK-293 cells stably expressing TRPM8 further confirmed direct binding of the steroid hormone, testosterone, to the TRPM8 protein. Applications of picomolar concentrations of testosterone to the primary human prostate cells, endogenously expressing TRPM8, elicited Ca(2+) responses and channel currents, and those were inhibited in the presence of TRPM8 antagonist, N-(2-aminoethyl)-N-(4-(benzyloxy)-3-methoxybenzyl)thiophene-2-carboxamide hydrochloride. These results indicate that the TRPM8 channel is physically associated with testosterone and suggest that, in addition to a genomic role, testosterone plays a role in direct regulation of the TRPM8 channel function.
Keywords: Androgen; Calcium Channel; Cold and Menthol Receptor TRPM8; Ion Channel; Testosterone; Transient Receptor Potential Channels (TRP Channels); Transient Receptor Potential Melastatin 8 Channel.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Bautista D. M., Siemens J., Glazer J. M., Tsuruda P. R., Basbaum A. I., Stucky C. L., Jordt S.-E., Julius D. (2007) The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448, 204–208 - PubMed
-
- Dhaka A., Murray A. N., Mathur J., Earley T. J., Petrus M. J., Patapoutian A. (2007) TRPM8 is required for cold sensation in mice. Neuron 54, 371–378 - PubMed
-
- Colburn R. W., Lubin M. L., Stone D. J., Jr., Wang Y., Lawrence D., D'Andrea M. R., Brandt M. R., Liu Y., Flores C. M., Qin N. (2007) Attenuated cold sensitivity in TRPM8 null mice. Neuron 54, 379–386 - PubMed
-
- McKemy D. D., Neuhausser W. M., Julius D. (2002) Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416, 52–58 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

