The TRPM8 protein is a testosterone receptor: II. Functional evidence for an ionotropic effect of testosterone on TRPM8
- PMID: 25480785
- PMCID: PMC4316998
- DOI: 10.1074/jbc.M114.610873
The TRPM8 protein is a testosterone receptor: II. Functional evidence for an ionotropic effect of testosterone on TRPM8
Abstract
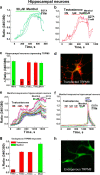
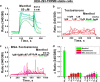
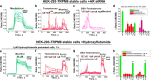
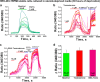
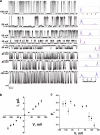
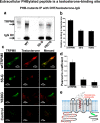
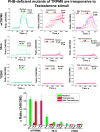
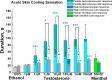
Testosterone is a key steroid hormone in the development of male reproductive tissues and the regulation of the central nervous system. The rapid signaling mechanism induced by testosterone affects numerous behavioral traits, including sexual drive, aggressiveness, and fear conditioning. However, the currently identified testosterone receptor(s) is not believed to underlie the fast signaling, suggesting an orphan pathway. Here we report that an ion channel from the transient receptor potential family, TRPM8, commonly known as the cold and menthol receptor is the major component of testosterone-induced rapid actions. Using cultured and primary cell lines along with the purified TRPM8 protein, we demonstrate that testosterone directly activates TRPM8 channel at low picomolar range. Specifically, testosterone induced TRPM8 responses in primary human prostate cells, PC3 prostate cancer cells, dorsal root ganglion neurons, and hippocampal neurons. Picomolar concentrations of testosterone resulted in full openings of the purified TRPM8 channel in planar lipid bilayers. Furthermore, acute applications of testosterone on human skin elicited a cooling sensation. Our data conclusively demonstrate that testosterone is an endogenous and highly potent agonist of TRPM8, suggesting a role of TRPM8 channels well beyond their well established function in somatosensory neurons. This discovery may further imply TRPM8 channel function in testosterone-dependent behavioral traits.
Keywords: Androgen; Androgen Receptor; Calcium Channel; Cold and Menthol Receptor TRPM8; Ion Channel; Testosterone; Transient Receptor Potential Channels (TRP Channels).
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
-
- Beato M., Chávez S., Truss M. (1996) Transcriptional regulation by steroid hormones. Steroids 61, 240–251 - PubMed
-
- Bruck N., Bastien J., Bour G., Tarrade A., Plassat J. L., Bauer A., Adam-Stitah S., Rochette-Egly C. (2005) Phosphorylation of the retinoid X receptor at the ω loop modulates the expression of retinoic-acid-target genes with a promoter context specificity. Cell. Signal. 17, 1229–1239 - PubMed
-
- Wierman M. E. (2007) Sex steroid effects at target tissues: mechanisms of action. Adv. Physiol. Educ. 31, 26–33 - PubMed
-
- Wehling M. (1997) Specific, nongenomic actions of steroid hormones. Annu. Rev. Physiol. 59, 365–393 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

