Lysosomal two-pore channel subtype 2 (TPC2) regulates skeletal muscle autophagic signaling
- PMID: 25480788
- PMCID: PMC4319008
- DOI: 10.1074/jbc.M114.608471
Lysosomal two-pore channel subtype 2 (TPC2) regulates skeletal muscle autophagic signaling
Abstract
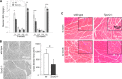
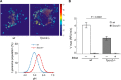
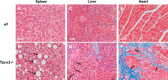
Postnatal skeletal muscle mass is regulated by the balance between anabolic protein synthesis and catabolic protein degradation, and muscle atrophy occurs when protein homeostasis is disrupted. Autophagy has emerged as critical in clearing dysfunctional organelles and thus in regulating protein turnover. Here we show that endolysosomal two-pore channel subtype 2 (TPC2) contributes to autophagy signaling and protein homeostasis in skeletal muscle. Muscles derived from Tpcn2(-/-) mice exhibit an atrophic phenotype with exacerbated autophagy under starvation. Compared with wild types, animals lacking TPC2 demonstrated an enhanced autophagy flux characterized by increased accumulation of autophagosomes upon combined stress induction by starvation and colchicine treatment. In addition, deletion of TPC2 in muscle caused aberrant lysosomal pH homeostasis and reduced lysosomal protease activity. Association between mammalian target of rapamycin and TPC2 was detected in skeletal muscle, allowing for appropriate adjustments to cellular metabolic states and subsequent execution of autophagy. TPC2 therefore impacts mammalian target of rapamycin reactivation during the process of autophagy and contributes to maintenance of muscle homeostasis.
Keywords: Autophagy; Calcium Channel; Lysosome; Muscle Atrophy; Protein Turnover.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Rennie M. J., Wackerhage H., Spangenburg E. E., Booth F. W. (2004) Control of the size of the human muscle mass. Annu. Rev. Physiol. 66, 799–828 - PubMed
-
- Schiaffino S., Dyar K. A., Ciciliot S., Blaauw B., Sandri M. (2013) Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 280, 4294–4314 - PubMed
-
- Choi A. M., Ryter S. W., Levine B. (2013) Autophagy in human health and disease. N. Engl. J. Med. 368, 651–662 - PubMed
-
- Sandri M. (2010) Autophagy in skeletal muscle. FEBS Lett. 584, 1411–1416 - PubMed
-
- Mizushima N., Komatsu M. (2011) Autophagy: renovation of cells and tissues. Cell 147, 728–741 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

