Ebola virus (EBOV) infection: Therapeutic strategies
- PMID: 25481298
- PMCID: PMC7110990
- DOI: 10.1016/j.bcp.2014.11.008
Ebola virus (EBOV) infection: Therapeutic strategies
Abstract
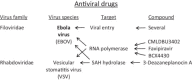
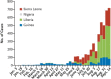
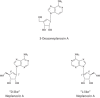
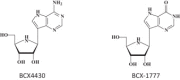
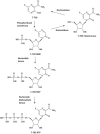
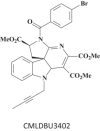
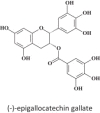
Within less than a year after its epidemic started (in December 2013) in Guinea, Ebola virus (EBOV), a member of the filoviridae, has spread over a number of West-African countries (Guinea, Sierra Leone and Liberia) and gained allures that have been unprecedented except by human immunodeficiency virus (HIV). Although EBOV is highly contagious and transmitted by direct contact with body fluids, it could be counteracted by the adequate chemoprophylactic and -therapeutic interventions: vaccines, antibodies, siRNAs (small interfering RNAs), interferons and chemical substances, i.e. neplanocin A derivatives (i.e. 3-deazaneplanocin A), BCX4430, favipiravir (T-705), endoplasmic reticulum (ER) α-glucosidase inhibitors and a variety of compounds that have been found to inhibit EBOV infection blocking viral entry or by a mode of action that still has to be resolved. Much has to be learned from the mechanism of action of the compounds active against VSV (vesicular stomatitis virus), a virus belonging to the rhabdoviridae, that in its mode of replication could be exemplary for the replication of filoviridae.
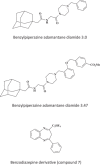
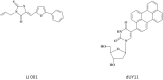
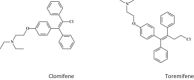
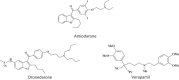
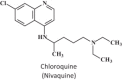
Keywords: 3-Deazaneplanocin A; 3-Deazaneplanocin A (PubChem CID: 73087); Amiodarone (PubChem CID: 2157); BCX-1777 (PubChem CID: 11493192); BCX4430; BCX4430 (PubChem CID: 69211190); Chloroquine (PubChem CID: 2719); Clomifene (PubChem CID: 1548953); Dronedarone (PubChem CID: 208898); Ebola; FGI-103 (PubChem CID: 5477931); Favipiravir; Favipiravir (PubChem CID: 492405); Filoviridae; LJ 001 (PubChem CID: 49777349); NSC62914 (PubChem CID: 66662); VSV (vesicular stomatitis virus); Verapamil (PubChem CID: 2520); dUY11 (PubChem CID: 24771429).
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures


References
-
- Gatherer D. The 2014 Ebola virus disease outbreak in West Africa. J Gen Virol. 2014;95:1619–1624. - PubMed
-
- Briand S., Bertherat E., Cox P., Formenty P., Kieny M.P., Myhre J.K. The international ebola emergency. N Engl J Med. 2014;371:1180–1183. - PubMed
-
- Vogel G. Infectious disease. Are bats spreading Ebola across sub-Saharan Africa? Science. 2014;344:140. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

