Independent modulation of contractile performance by cardiac troponin I Ser43 and Ser45 in the dynamic sarcomere
- PMID: 25481661
- PMCID: PMC4301988
- DOI: 10.1016/j.yjmcc.2014.11.022
Independent modulation of contractile performance by cardiac troponin I Ser43 and Ser45 in the dynamic sarcomere
Abstract
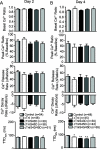
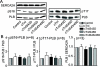
Protein kinase C (PKC) targets cardiac troponin I (cTnI) S43/45 for phosphorylation in addition to other residues. During heart failure, cTnI S43/45 phosphorylation is elevated, and yet there is ongoing debate about its functional role due, in part, to the emergence of complex phenotypes in animal models. The individual functional influences of phosphorylated S43 and S45 also are not yet known. The present study utilizes viral gene transfer of cTnI with phosphomimetic S43D and/or S45D substitutions to evaluate their individual and combined influences on function in intact adult cardiac myocytes. Partial replacement (≤40%) with either cTnIS43D or cTnIS45D reduced the amplitude of contraction, and cTnIS45D slowed contraction and relaxation rates, while there were no significant changes in function with cTnIS43/45D. More extensive replacement (≥70%) with cTnIS43D, cTnIS45D, and cTnIS43/45D each reduced the amplitude of contraction. Additional experiments also showed cTnIS45D reduced myofilament Ca(2+) sensitivity of tension. At the same time, shortening rates returned toward control values with cTnIS45D and the later stages of relaxation also became accelerated in myocytes expressing cTnIS43D and/or S45D. Further studies demonstrated this behavior coincided with adaptive changes in myofilament protein phosphorylation. Taken together, the results observed in myocytes expressing cTnIS43D and/or S45D suggest these 2 residues reduce function via independent mechanism(s). The changes in function associated with the onset of adaptive myofilament signaling suggest the sarcomere is capable of fine tuning PKC-mediated cTnIS43/45 phosphorylation and contractile performance. This modulatory behavior also provides insight into divergent phenotypes reported in animal models with cTnI S43/45 phosphomimetic substitutions.
Keywords: Myocyte; Myofilament; Phosphorylation; Protein kinase C; Troponin.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures






Similar articles
-
Troponin I modulation of cardiac performance: Plasticity in the survival switch.Arch Biochem Biophys. 2019 Mar 30;664:9-14. doi: 10.1016/j.abb.2019.01.025. Epub 2019 Jan 23. Arch Biochem Biophys. 2019. PMID: 30684464 Free PMC article. Review.
-
Functionally conservative substitutions at cardiac troponin I S43/45.Arch Biochem Biophys. 2016 Jul 1;601:42-7. doi: 10.1016/j.abb.2016.02.002. Epub 2016 Feb 8. Arch Biochem Biophys. 2016. PMID: 26869200 Free PMC article.
-
Myofilament incorporation and contractile function after gene transfer of cardiac troponin I Ser43/45Ala.Arch Biochem Biophys. 2013 Jul 1;535(1):49-55. doi: 10.1016/j.abb.2012.12.021. Epub 2013 Jan 11. Arch Biochem Biophys. 2013. PMID: 23318976 Free PMC article.
-
Functional communication between PKC-targeted cardiac troponin I phosphorylation sites.Arch Biochem Biophys. 2017 Aug 1;627:1-9. doi: 10.1016/j.abb.2017.05.019. Epub 2017 Jun 3. Arch Biochem Biophys. 2017. PMID: 28587770 Free PMC article.
-
Regulation of cardiac contractile function by troponin I phosphorylation.Cardiovasc Res. 2005 Apr 1;66(1):12-21. doi: 10.1016/j.cardiores.2004.12.022. Cardiovasc Res. 2005. PMID: 15769444 Review.
Cited by
-
Extracellular Histone-Induced Protein Kinase C Alpha Activation and Troponin Phosphorylation Is a Potential Mechanism of Cardiac Contractility Depression in Sepsis.Int J Mol Sci. 2023 Feb 6;24(4):3225. doi: 10.3390/ijms24043225. Int J Mol Sci. 2023. PMID: 36834636 Free PMC article.
-
Troponin I modulation of cardiac performance: Plasticity in the survival switch.Arch Biochem Biophys. 2019 Mar 30;664:9-14. doi: 10.1016/j.abb.2019.01.025. Epub 2019 Jan 23. Arch Biochem Biophys. 2019. PMID: 30684464 Free PMC article. Review.
-
Secondary phosphorylation in myocytes expressing FLAG-tagged and non-tagged phospho-mimetic cardiac troponin I.Data Brief. 2017 Oct 5;15:562-566. doi: 10.1016/j.dib.2017.09.066. eCollection 2017 Dec. Data Brief. 2017. PMID: 29071293 Free PMC article.
-
Functionally conservative substitutions at cardiac troponin I S43/45.Arch Biochem Biophys. 2016 Jul 1;601:42-7. doi: 10.1016/j.abb.2016.02.002. Epub 2016 Feb 8. Arch Biochem Biophys. 2016. PMID: 26869200 Free PMC article.
-
The continuing evolution of cardiac troponin I biomarker analysis: from protein to proteoform.Expert Rev Proteomics. 2017 Nov;14(11):973-986. doi: 10.1080/14789450.2017.1387054. Epub 2017 Oct 16. Expert Rev Proteomics. 2017. PMID: 28984473 Free PMC article. Review.
References
-
- Layland J, Solaro RJ, Shah AM. Regulation of cardiac contractile function by troponin I phosphorylation. Cardiovasc Res. 2005;66:12–21. - PubMed
-
- Westfall MV. Post-translational modification of troponin. In: Jin J-P, editor. Troponin: Regulator of muscle contraction. Nova Science; New York: 2013. pp. 163–203.
-
- Yasuda S, Coutu P, Sadayappan S, Robbins J, Metzger JM. Cardiac transgenic and gene transfer strategies converge to support an important role for troponin I in regulating relaxation in cardiac myocytes. Circ Res. 2007;101:377–386. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

