Epigenetic mechanisms in diabetic complications and metabolic memory
- PMID: 25481708
- PMCID: PMC4324095
- DOI: 10.1007/s00125-014-3462-y
Epigenetic mechanisms in diabetic complications and metabolic memory
Abstract
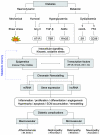
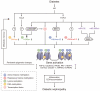
The incidence of diabetes and its associated micro- and macrovascular complications is greatly increasing worldwide. The most prevalent vascular complications of both type 1 and type 2 diabetes include nephropathy, retinopathy, neuropathy and cardiovascular diseases. Evidence suggests that both genetic and environmental factors are involved in these pathologies. Clinical trials have underscored the beneficial effects of intensive glycaemic control for preventing the progression of complications. Accumulating evidence suggests a key role for epigenetic mechanisms such as DNA methylation, histone post-translational modifications in chromatin, and non-coding RNAs in the complex interplay between genes and the environment. Factors associated with the pathology of diabetic complications, including hyperglycaemia, growth factors, oxidant stress and inflammatory factors can lead to dysregulation of these epigenetic mechanisms to alter the expression of pathological genes in target cells such as endothelial, vascular smooth muscle, retinal and cardiac cells, without changes in the underlying DNA sequence. Furthermore, long-term persistence of these alterations to the epigenome may be a key mechanism underlying the phenomenon of 'metabolic memory' and sustained vascular dysfunction despite attainment of glycaemic control. Current therapies for most diabetic complications have not been fully efficacious, and hence a study of epigenetic mechanisms that may be involved is clearly warranted as they can not only shed novel new insights into the pathology of diabetic complications, but also lead to the identification of much needed new drug targets. In this review, we highlight the emerging role of epigenetics and epigenomics in the vascular complications of diabetes and metabolic memory.
Figures


References
-
- Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–2581. - PubMed
-
- Ziyadeh FN, Sharma K. Overview: combating diabetic nephropathy. Journal of the American Society of Nephrology : JASN. 2003;14:1355–1357. - PubMed
-
- Fong DS, Aiello L, Gardner TW, et al. Diabetic retinopathy. Diabetes care. 2003;26:226–229. - PubMed
-
- Natarajan R, Nadler JL. Lipid inflammatory mediators in diabetic vascular disease. Arterioscler Thromb Vasc Biol. 2004;24:1542–1548. - PubMed
-
- Vincent AM, Calabek B, Roberts L, Feldman EL. Biology of diabetic neuropathy. Handbook of clinical neurology. 2013;115:591–606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

