Circulating antibodies against Plasmodium falciparum histidine-rich proteins 2 interfere with antigen detection by rapid diagnostic tests
- PMID: 25481825
- PMCID: PMC4295572
- DOI: 10.1186/1475-2875-13-480
Circulating antibodies against Plasmodium falciparum histidine-rich proteins 2 interfere with antigen detection by rapid diagnostic tests
Abstract
Background: Rapid diagnostic tests (RDTs) for detection of Plasmodium falciparum infection that target P. falciparum histidine-rich protein 2 (PfHRP2), a protein that circulates in the blood of patients infected with this species of malaria, are widely used to guide case management. Understanding determinants of PfHRP2 availability in circulation is therefore essential to understanding the performance of PfHRP2-detecting RDTs.
Methods: The possibility that pre-formed host anti-PfHRP2 antibodies may block target antigen detection, thereby causing false negative test results was investigated in this study.
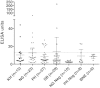
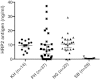
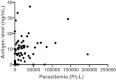
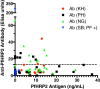
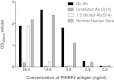
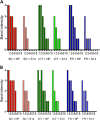
Results: Anti-PfHRP2 antibodies were detected in 19/75 (25%) of plasma samples collected from patients with acute malaria from Cambodia, Nigeria and the Philippines, as well as in 3/28 (10.7%) asymptomatic Solomon Islands residents. Pre-incubation of plasma samples from subjects with high-titre anti-PfHRP2 antibodies with soluble PfHRP2 blocked the detection of the target antigen on two of the three brands of RDTs tested, leading to false negative results. Pre-incubation of the plasma with intact parasitized erythrocytes resulted in a reduction of band intensity at the highest parasite density, and a reduction of lower detection threshold by ten-fold on all three brands of RDTs tested.
Conclusions: These observations indicate possible reduced sensitivity for diagnosis of P. falciparum malaria using PfHRP2-detecting RDTs among people with high levels of specific antibodies and low density infection, as well as possible interference with tests configured to detect soluble PfHRP2 in saliva or urine samples. Further investigations are required to assess the impact of pre-formed anti-PfHRP2 antibodies on RDT performance in different transmission settings.
Figures
References
-
- WHO . Guidelines for the Treatment of Malaria. 2. Geneva: World Health Organization; 2010. - PubMed
-
- WHO . World Malaria Report 2013. Geneva: World Health Organization; 2013.
-
- Stow NW, Torrens JK, Walker J. An assessment of the accuracy of clinical diagnosis, local microscopy and a rapid immunochromatographic card test in comparison with expert microscopy in the diagnosis of malaria in rural Kenya. Trans R Soc Trop Med Hyg. 1999;93:519–520. doi: 10.1016/S0035-9203(99)90359-0. - DOI - PubMed
-
- Endeshaw T, Gebre T, Ngondi J, Graves PM, Shargie EB, Ejigsemahu Y, Ayele B, Yohannes G, Teferi T, Messele A, Zerihun M, Genet A, Mosher AW, Emerson PM, Richards FO. Evaluation of light microscopy and rapid diagnostic test for the detection of malaria under operational field conditions: a household survey in Ethiopia. Malar J. 2008;7:118. doi: 10.1186/1475-2875-7-118. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

