Pathogenesis of nasal polyposis
- PMID: 25482020
- PMCID: PMC4422388
- DOI: 10.1111/cea.12472
Pathogenesis of nasal polyposis
Abstract
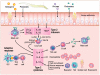
Chronic rhinosinusitis with nasal polyps (CRSwNP) is a complex inflammatory condition that affects a large proportion of the population world-wide and is associated with high cost of management and significant morbidity. Yet, there is a lack of population-based epidemiologic studies using current definitions of CRSwNP, and the mechanisms that drive pathogenesis in this disease remain unclear. In this review, we summarize the current evidence for the plethora of factors that likely contribute to CRSwNP pathogenesis. Defects in the innate function of the airway epithelial barrier, including diminished expression of antimicrobial products and loss of barrier integrity, combined with colonization by fungi and bacteria likely play a critical role in the development of chronic inflammation in CRSwNP. This chronic inflammation is characterized by elevated expression of many key inflammatory cytokines and chemokines, including IL-5, thymic stromal lymphopoietin and CCL11, that help to initiate and perpetuate this chronic inflammatory response. Together, these factors likely combine to drive the influx of a variety of immune cells, including eosinophils, mast cells, group 2 innate lymphoid cells and lymphocytes, which participate in the chronic inflammatory response within the nasal polyps. Importantly, however, future studies are needed to demonstrate the necessity and sufficiency of these potential drivers of disease in CRSwNP. In addition to the development of new tools and models to aid mechanistic studies, the field of CRSwNP research also needs the type of robust epidemiologic data that has served the asthma community so well. Given the high prevalence, costs and morbidity, there is a great need for continued research into CRS that could facilitate the development of novel therapeutic strategies to improve treatment for patients who suffer from this disease.
© 2014 John Wiley & Sons Ltd.
Figures


Similar articles
-
Pathophysiology of chronic rhinosinusitis with nasal polyp.Am J Rhinol Allergy. 2011 Sep-Oct;25(5):285-90. doi: 10.2500/ajra.2011.25.3680. Am J Rhinol Allergy. 2011. PMID: 22186239 Review.
-
The antimicrobial protein short palate, lung, and nasal epithelium clone 1 (SPLUNC1) is differentially modulated in eosinophilic and noneosinophilic chronic rhinosinusitis with nasal polyps.J Allergy Clin Immunol. 2014 Feb;133(2):420-8. doi: 10.1016/j.jaci.2013.09.052. Epub 2013 Dec 15. J Allergy Clin Immunol. 2014. PMID: 24342548
-
Airway Inflammation in Chronic Rhinosinusitis with Nasal Polyps and Asthma: The United Airways Concept Further Supported.PLoS One. 2015 Jul 1;10(7):e0127228. doi: 10.1371/journal.pone.0127228. eCollection 2015. PLoS One. 2015. PMID: 26132710 Free PMC article.
-
Group 2 innate lymphoid cells (ILC2s) are increased in chronic rhinosinusitis with nasal polyps or eosinophilia.Clin Exp Allergy. 2015 Feb;45(2):394-403. doi: 10.1111/cea.12462. Clin Exp Allergy. 2015. PMID: 25429730
-
Proallergic cytokines and group 2 innate lymphoid cells in allergic nasal diseases.Allergol Int. 2015 Jul;64(3):235-40. doi: 10.1016/j.alit.2014.12.008. Epub 2015 Feb 10. Allergol Int. 2015. PMID: 26117254 Review.
Cited by
-
Expression of E-prostanoid receptors in nasal polyp tissues of smoking and nonsmoking patients with chronic rhinosinusitis.PLoS One. 2018 Jul 24;13(7):e0200989. doi: 10.1371/journal.pone.0200989. eCollection 2018. PLoS One. 2018. PMID: 30040868 Free PMC article.
-
Chinese Society of Allergy and Chinese Society of Otorhinolaryngology-Head and Neck Surgery Guideline for Chronic Rhinosinusitis.Allergy Asthma Immunol Res. 2020 Mar;12(2):176-237. doi: 10.4168/aair.2020.12.2.176. Allergy Asthma Immunol Res. 2020. PMID: 32009319 Free PMC article. Review.
-
CD133, a Progenitor Cell Marker, is Reduced in Nasal Polyposis and Showed Significant Correlations with TGF-β1 and IL-8.Int Arch Otorhinolaryngol. 2021 Jun 25;26(1):e091-e096. doi: 10.1055/s-0041-1726043. eCollection 2022 Jan. Int Arch Otorhinolaryngol. 2021. PMID: 35096164 Free PMC article.
-
Interactions between dendritic cells and T lymphocytes in pathogenesis of nasal polyps.Exp Ther Med. 2018 Jun;15(6):5167-5172. doi: 10.3892/etm.2018.6128. Epub 2018 May 3. Exp Ther Med. 2018. PMID: 29904400 Free PMC article.
-
Biomarkers in the evaluation and management of chronic rhinosinusitis with nasal polyposis.Eur Arch Otorhinolaryngol. 2017 Oct;274(10):3559-3566. doi: 10.1007/s00405-017-4547-2. Epub 2017 Apr 1. Eur Arch Otorhinolaryngol. 2017. PMID: 28365802 Review.
References
-
- WJ Fokkens, Lund VJ, Mullol J, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012;50:1–12. - PubMed
-
- Jarvis D, Newson R, Lotvall J, et al. Asthma in adults and its association with chronic rhinosinusitis: the GA2LEN survey in Europe. Allergy. 2012;67:91–8. - PubMed
-
- Kim YS, Kim NH, Seong SY, Kim KR, Lee GB, Kim KS. Prevalence and risk factors of chronic rhinosinusitis in Korea. Am J Rhinol Allergy. 2011;25:117–21. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

