Multiple glass transitions and freezing events of aqueous citric acid
- PMID: 25482069
- PMCID: PMC4434526
- DOI: 10.1021/jp510331h
Multiple glass transitions and freezing events of aqueous citric acid
Abstract
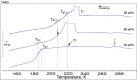
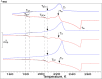
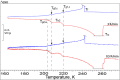
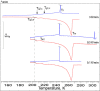
Calorimetric and optical cryo-microscope measurements of 10-64 wt % citric acid (CA) solutions subjected to moderate (3 K/min) and slow (0.5 and 0.1 K/min) cooling/warming rates and also to quenching/moderate warming between 320 and 133 K are presented. Depending on solution concentration and cooling rate, the obtained thermograms show one freezing event and from one to three liquid-glass transitions upon cooling and from one to six liquid-glass and reverse glass-liquid transitions, one or two freezing events, and one melting event upon warming of frozen/glassy CA/H2O. The multiple freezing events and glass transitions pertain to the mother CA/H2O solution itself and two freeze-concentrated solution regions, FCS1 and FCS2, of different concentrations. The FCS1 and FCS2 (or FCS22) are formed during the freezing of CA/H2O upon cooling and/or during the freezing upon warming of partly glassy or entirely glassy mother CA/H2O. The formation of two FCS1 and FCS22 regions during the freezing upon warming to our best knowledge has never been reported before. Using an optical cryo-microscope, we are able to observe the formation of a continuous ice framework (IF) and its morphology and reciprocal distribution of IF/(FCS1 + FCS2). Our results provide a new look at the freezing and glass transition behavior of aqueous solutions and can be used for the optimization of lyophilization and freezing of foods and biopharmaceutical formulations, among many other applications where freezing plays a crucial role.
Figures






References
-
- Nordmann C. E.; Weldon A. S.; Patterson A. L. X-Ray Crystal Analysis of the Substrates of Aconitase. II. Anhydrous Citric Acid. Acta Crystallogr. 1960, 13, 418–426.
-
- Mullin J. W.; Leci C. L. Evidence of Molecular Cluster Formation in Supersaturated Solutions of Citric Acid. Philos. Mag. 1969, 19, 1075–1077.
-
- Mullin J. W.; Leci C. L. Some Nucleation Characteristics of Aqueous Citric Acid Solutions. J. Cryst. Growth 1969, 5, 75–76.
-
- Timko R. J.; Lordi N. G. The Effect of Thermal History on the Transition Temperature of Citric Acid Glass. J. Pharm. Sci. 1979, 71, 1185–1186. - PubMed
-
- Maltini E.; Anese M.; Shtylla I. State Diagram of Some Organic Acid-Water Systems of Interest in Food. Cryo Lett. 1997, 18, 263–268.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

