PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma
- PMID: 25482239
- PMCID: PMC4348009
- DOI: 10.1056/NEJMoa1411087
PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma
Abstract
Background: Preclinical studies suggest that Reed-Sternberg cells exploit the programmed death 1 (PD-1) pathway to evade immune detection. In classic Hodgkin's lymphoma, alterations in chromosome 9p24.1 increase the abundance of the PD-1 ligands, PD-L1 and PD-L2, and promote their induction through Janus kinase (JAK)-signal transducer and activator of transcription (STAT) signaling. We hypothesized that nivolumab, a PD-1-blocking antibody, could inhibit tumor immune evasion in patients with relapsed or refractory Hodgkin's lymphoma.
Methods: In this ongoing study, 23 patients with relapsed or refractory Hodgkin's lymphoma that had already been heavily treated received nivolumab (at a dose of 3 mg per kilogram of body weight) every 2 weeks until they had a complete response, tumor progression, or excessive toxic effects. Study objectives were measurement of safety and efficacy and assessment of the PDL1 and PDL2 (also called CD274 and PDCD1LG2, respectively) loci and PD-L1 and PD-L2 protein expression.
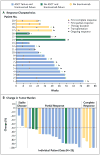
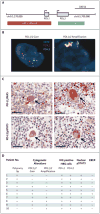
Results: Of the 23 study patients, 78% were enrolled in the study after a relapse following autologous stem-cell transplantation and 78% after a relapse following the receipt of brentuximab vedotin. Drug-related adverse events of any grade and of grade 3 occurred in 78% and 22% of patients, respectively. An objective response was reported in 20 patients (87%), including 17% with a complete response and 70% with a partial response; the remaining 3 patients (13%) had stable disease. The rate of progression-free survival at 24 weeks was 86%; 11 patients were continuing to participate in the study. Reasons for discontinuation included stem-cell transplantation (in 6 patients), disease progression (in 4 patients), and drug toxicity (in 2 patients). Analyses of pretreatment tumor specimens from 10 patients revealed copy-number gains in PDL1 and PDL2 and increased expression of these ligands. Reed-Sternberg cells showed nuclear positivity of phosphorylated STAT3, indicative of active JAK-STAT signaling.
Conclusions: Nivolumab had substantial therapeutic activity and an acceptable safety profile in patients with previously heavily treated relapsed or refractory Hodgkin's lymphoma. (Funded by Bristol-Myers Squibb and others; ClinicalTrials.gov number, NCT01592370.).
Conflict of interest statement
No other potential conflict of interest relevant to this article was reported.
Figures
Comment in
-
Release the hounds! Activating the T-cell response to cancer.N Engl J Med. 2015 Jan 22;372(4):374-5. doi: 10.1056/NEJMe1413488. Epub 2014 Dec 6. N Engl J Med. 2015. PMID: 25482238 No abstract available.
-
Haematological cancer: PD-1 blockade: opening the door to attack.Nat Rev Clin Oncol. 2015 Feb;12(2):65. doi: 10.1038/nrclinonc.2014.227. Epub 2014 Dec 23. Nat Rev Clin Oncol. 2015. PMID: 25533946 No abstract available.
-
Nivolumab shows clinical activity in Hodgkin's lymphoma.Lancet Oncol. 2015 Mar;16(3):e108. doi: 10.1016/S1470-2045(15)70024-0. Epub 2015 Jan 30. Lancet Oncol. 2015. PMID: 25639369 No abstract available.
References
-
- Weber J. Immune checkpoint proteins: a new therapeutic paradigm for cancer — preclinical background: CTLA-4 and PD-1 blockade. Semin Oncol. 2010;37:430–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
