The diverse chemistry of cytochrome P450 17A1 (P450c17, CYP17A1)
- PMID: 25482340
- PMCID: PMC4456341
- DOI: 10.1016/j.jsbmb.2014.11.026
The diverse chemistry of cytochrome P450 17A1 (P450c17, CYP17A1)
Abstract
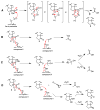
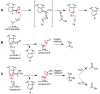
The steroid hydroxylation and carbon-carbon bond cleavage activities of cytochrome P450 17A1 (CYP17A1) are responsible for the production of glucocorticoids and androgens, respectively. The inhibition of androgen synthesis is an important strategy to treat androgen-dependent prostate cancer. We discuss the different enzymatic activities towards the various substrates of CYP17A1, demonstrating its promiscuity. Additionally, a novel interhelical interaction is proposed between the F-G loop and the B'-helix to explain the 16α-hydroxylase activity of human CYP17A1 with progesterone as the substrate. The techniques used by biochemists to study this important enzyme are also summarized. This article is part of a Special Issue entitled 'Steroid/Sterol signaling'.
Keywords: 17,20-Lyase; 17-Hydroxylase; Androgen; Cytochrome P450; Hypertension; Metabolic switching; Steroidogenesis.
Copyright © 2014. Published by Elsevier Ltd.
Figures





Similar articles
-
Epoxidation activities of human cytochromes P450c17 and P450c21.Biochemistry. 2014 Dec 9;53(48):7531-40. doi: 10.1021/bi5011865. Epub 2014 Nov 25. Biochemistry. 2014. PMID: 25386927 Free PMC article.
-
Structural insights into the function of steroidogenic cytochrome P450 17A1.Mol Cell Endocrinol. 2017 Feb 5;441:68-75. doi: 10.1016/j.mce.2016.08.035. Epub 2016 Aug 24. Mol Cell Endocrinol. 2017. PMID: 27566228 Free PMC article. Review.
-
Structures of human steroidogenic cytochrome P450 17A1 with substrates.J Biol Chem. 2014 Nov 21;289(47):32952-64. doi: 10.1074/jbc.M114.610998. Epub 2014 Oct 9. J Biol Chem. 2014. PMID: 25301938 Free PMC article.
-
Steroid 17α-hydroxylase/17, 20-lyase (cytochrome P450 17A1).Methods Enzymol. 2023;689:39-63. doi: 10.1016/bs.mie.2023.04.001. Epub 2023 Apr 25. Methods Enzymol. 2023. PMID: 37802581 Free PMC article.
-
Role of cytochrome b5 in the modulation of the enzymatic activities of cytochrome P450 17α-hydroxylase/17,20-lyase (P450 17A1).J Steroid Biochem Mol Biol. 2017 Jun;170:2-18. doi: 10.1016/j.jsbmb.2016.02.033. Epub 2016 Mar 11. J Steroid Biochem Mol Biol. 2017. PMID: 26976652 Review.
Cited by
-
Metal-Free Catalysis in C-C Single-Bond Cleavage: Achievements and Prospects.Top Curr Chem (Cham). 2022 Aug 11;380(5):38. doi: 10.1007/s41061-022-00393-7. Top Curr Chem (Cham). 2022. PMID: 35951267 Review.
-
Knockdown of CYP19A1 in Buffalo Follicular Granulosa Cells Results in Increased Progesterone Secretion and Promotes Cell Proliferation.Front Vet Sci. 2020 Sep 25;7:539496. doi: 10.3389/fvets.2020.539496. eCollection 2020. Front Vet Sci. 2020. PMID: 33102564 Free PMC article.
-
Triphenyltin Chloride Delays Leydig Cell Maturation During Puberty in Rats.Front Pharmacol. 2018 Aug 10;9:833. doi: 10.3389/fphar.2018.00833. eCollection 2018. Front Pharmacol. 2018. PMID: 30147652 Free PMC article.
-
A synthesis of a rationally designed inhibitor of cytochrome P450 8B1, a therapeutic target to treat obesity.Steroids. 2022 Feb;178:108952. doi: 10.1016/j.steroids.2021.108952. Epub 2021 Dec 27. Steroids. 2022. PMID: 34968450 Free PMC article.
-
Mechanistic Scrutiny Identifies a Kinetic Role for Cytochrome b5 Regulation of Human Cytochrome P450c17 (CYP17A1, P450 17A1).PLoS One. 2015 Nov 20;10(11):e0141252. doi: 10.1371/journal.pone.0141252. eCollection 2015. PLoS One. 2015. PMID: 26587646 Free PMC article.
References
-
- Guengerich FP. Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicology. Chem Res Toxicol. 2001;14:611–650. - PubMed
-
- Rittle J, Green MT. Cytochrome P450 compound I: capture, characterization, and C-H bond activation kinetics. Science. 2010;330:933–937. - PubMed
-
- Hasseman CA, Kurumbail RG, Boddupalli SS, Peterson JA, Deisenhofer J. Structure and function of cytochrome P450: a comparative analysis of three crystal structures. Structure. 1995;3:41–62. - PubMed
-
- Hanukoglu I. Steroidogenic enzymes: structure, function, and role in regulation of steroid hormone biosynthesis. J Steroid Biochem Mol Biol. 1992;43:779–804. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

