The diverse chemistry of cytochrome P450 17A1 (P450c17, CYP17A1)
- PMID: 25482340
- PMCID: PMC4456341
- DOI: 10.1016/j.jsbmb.2014.11.026
The diverse chemistry of cytochrome P450 17A1 (P450c17, CYP17A1)
Abstract
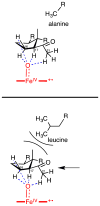
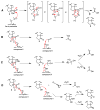
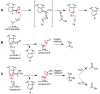
The steroid hydroxylation and carbon-carbon bond cleavage activities of cytochrome P450 17A1 (CYP17A1) are responsible for the production of glucocorticoids and androgens, respectively. The inhibition of androgen synthesis is an important strategy to treat androgen-dependent prostate cancer. We discuss the different enzymatic activities towards the various substrates of CYP17A1, demonstrating its promiscuity. Additionally, a novel interhelical interaction is proposed between the F-G loop and the B'-helix to explain the 16α-hydroxylase activity of human CYP17A1 with progesterone as the substrate. The techniques used by biochemists to study this important enzyme are also summarized. This article is part of a Special Issue entitled 'Steroid/Sterol signaling'.
Keywords: 17,20-Lyase; 17-Hydroxylase; Androgen; Cytochrome P450; Hypertension; Metabolic switching; Steroidogenesis.
Copyright © 2014. Published by Elsevier Ltd.
Figures





References
-
- Guengerich FP. Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicology. Chem Res Toxicol. 2001;14:611–650. - PubMed
-
- Rittle J, Green MT. Cytochrome P450 compound I: capture, characterization, and C-H bond activation kinetics. Science. 2010;330:933–937. - PubMed
-
- Hasseman CA, Kurumbail RG, Boddupalli SS, Peterson JA, Deisenhofer J. Structure and function of cytochrome P450: a comparative analysis of three crystal structures. Structure. 1995;3:41–62. - PubMed
-
- Hanukoglu I. Steroidogenic enzymes: structure, function, and role in regulation of steroid hormone biosynthesis. J Steroid Biochem Mol Biol. 1992;43:779–804. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

