Factor XI antisense oligonucleotide for prevention of venous thrombosis
- PMID: 25482425
- PMCID: PMC4367537
- DOI: 10.1056/NEJMoa1405760
Factor XI antisense oligonucleotide for prevention of venous thrombosis
Abstract
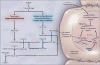
Background: Experimental data indicate that reducing factor XI levels attenuates thrombosis without causing bleeding, but the role of factor XI in the prevention of postoperative venous thrombosis in humans is unknown. FXI-ASO (ISIS 416858) is a second-generation antisense oligonucleotide that specifically reduces factor XI levels. We compared the efficacy and safety of FXI-ASO with those of enoxaparin in patients undergoing total knee arthroplasty.
Methods: In this open-label, parallel-group study, we randomly assigned 300 patients who were undergoing elective primary unilateral total knee arthroplasty to receive one of two doses of FXI-ASO (200 mg or 300 mg) or 40 mg of enoxaparin once daily. The primary efficacy outcome was the incidence of venous thromboembolism (assessed by mandatory bilateral venography or report of symptomatic events). The principal safety outcome was major or clinically relevant nonmajor bleeding.
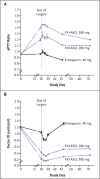
Results: Around the time of surgery, the mean (±SE) factor XI levels were 0.38±0.01 units per milliliter in the 200-mg FXI-ASO group, 0.20±0.01 units per milliliter in the 300-mg FXI-ASO group, and 0.93±0.02 units per milliliter in the enoxaparin group. The primary efficacy outcome occurred in 36 of 134 patients (27%) who received the 200-mg dose of FXI-ASO and in 3 of 71 patients (4%) who received the 300-mg dose of FXI-ASO, as compared with 21 of 69 patients (30%) who received enoxaparin. The 200-mg regimen was noninferior, and the 300-mg regimen was superior, to enoxaparin (P<0.001). Bleeding occurred in 3%, 3%, and 8% of the patients in the three study groups, respectively.
Conclusions: This study showed that factor XI contributes to postoperative venous thromboembolism; reducing factor XI levels in patients undergoing elective primary unilateral total knee arthroplasty was an effective method for its prevention and appeared to be safe with respect to the risk of bleeding. (Funded by Isis Pharmaceuticals; FXI-ASO TKA ClinicalTrials.gov number, NCT01713361.).
Figures

Comment in
-
Making (anti)sense of factor XI in thrombosis.N Engl J Med. 2015 Jan 15;372(3):277-8. doi: 10.1056/NEJMe1413874. Epub 2014 Dec 7. N Engl J Med. 2015. PMID: 25482334 No abstract available.
-
Anticoagulation therapy: Reducing factor XI with antisense oligonucleotides superior to endoxaparin for postoperative venous thromboembolism.Nat Rev Cardiol. 2015 Feb;12(2):66. doi: 10.1038/nrcardio.2014.214. Epub 2014 Dec 23. Nat Rev Cardiol. 2015. PMID: 25533800 No abstract available.
-
Factor XI antisense oligonucleotide for venous thrombosis.N Engl J Med. 2015 Apr 23;372(17):1672. doi: 10.1056/NEJMc1503223. N Engl J Med. 2015. PMID: 25901434 No abstract available.
-
Factor XI antisense oligonucleotide for venous thrombosis.N Engl J Med. 2015 Apr 23;372(17):1671. doi: 10.1056/NEJMc1503223. N Engl J Med. 2015. PMID: 25901435 No abstract available.
-
Factor XI antisense oligonucleotide for venous thrombosis.N Engl J Med. 2015 Apr 23;372(17):1671-2. doi: 10.1056/NEJMc1503223. N Engl J Med. 2015. PMID: 25901436 No abstract available.
References
-
- Zhang H, Löwenberg EC, Crosby JR, et al. Inhibition of the intrinsic coagulation pathway factor XI by antisense oligonucleotides: a novel antithrombotic strategy with lowered bleeding risk. Blood. 2010;116:4684–92. - PubMed
-
- Wang X, Smith PL, Hsu MY, et al. Effects of factor XI deficiency on ferric chloride-induced vena cava thrombosis in mice. J Thromb Haemost. 2006;4:1982–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
