Liver X receptors balance lipid stores in hepatic stellate cells through Rab18, a retinoid responsive lipid droplet protein
- PMID: 25482505
- PMCID: PMC4458237
- DOI: 10.1002/hep.27645
Liver X receptors balance lipid stores in hepatic stellate cells through Rab18, a retinoid responsive lipid droplet protein
Abstract
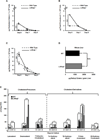
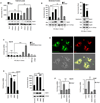
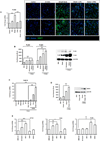
Liver X receptors (LXRs) are determinants of hepatic stellate cell (HSC) activation and liver fibrosis. Freshly isolated HSCs from Lxrαβ(-/-) mice have increased lipid droplet (LD) size, but the functional consequences of this are unknown. Our aim was to determine whether LXRs link cholesterol to retinoid storage in HSCs and how this impacts activation. Primary HSCs from Lxrαβ(-/-) and wild-type mice were profiled by gene array during in vitro activation. Lipid content was quantified by high-performance liquid chromatography and mass spectroscopy. Primary HSCs were treated with nuclear receptor ligands, transfected with small interfering RNA and plasmid constructs, and analyzed by immunocytochemistry. Lxrαβ(-/-) HSCs have increased cholesterol and retinyl esters. The retinoid increase drives intrinsic retinoic acid receptor signaling, and activation occurs more rapidly in Lxrαβ(-/-) HSCs. We identify Rab18 as a novel retinoic acid-responsive, LD-associated protein that helps mediate stellate cell activation. Rab18 mRNA, protein, and membrane insertion increase during activation. Both Rab18 guanosine triphosphatase activity and isoprenylation are required for stellate cell LD loss and induction of activation markers. These phenomena are accelerated in Lxrαβ(-/-) HSCs, where there is greater retinoic acid flux. Conversely, Rab18 knockdown retards LD loss in culture and blocks activation, just like the functional mutants. Rab18 is also induced with acute liver injury in vivo.
Conclusion: Retinoid and cholesterol metabolism are linked in stellate cells by the LD-associated protein Rab18. Retinoid overload helps explain the profibrotic phenotype of Lxrαβ(-/-) mice, and we establish a pivotal role for Rab18 GTPase activity and membrane insertion in wild-type stellate cell activation. Interference with Rab18 may have significant therapeutic benefit in ameliorating liver fibrosis.
© 2015 by the American Association for the Study of Liver Diseases.
Figures




References
-
- Kluwe J, Wongsiriroj N, Troeger JS, Gwak GY, Dapito DH, Pradere JP, Jiang H, et al. Absence of hepatic stellate cell retinoid lipid droplets does not enhance hepatic fibrosis but decreases hepatic carcinogenesis. Gut. 2011;60:1260–1268. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
