Pressure-assisted dissociation and degradation of "proteinase K-resistant" fibrils prepared by seeding with scrapie-infected hamster prion protein
- PMID: 25482603
- PMCID: PMC4601478
- DOI: 10.4161/pri.32081
Pressure-assisted dissociation and degradation of "proteinase K-resistant" fibrils prepared by seeding with scrapie-infected hamster prion protein
Abstract
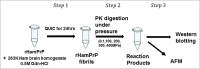
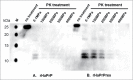
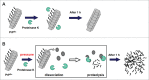
The crucial step for the fatal neurodegenerative prion diseases involves the conversion of a normal cellular protein, PrP(C), into a fibrous pathogenic form, PrP(Sc), which has an unusual stability against heat and resistance against proteinase K digestion. A successful challenge to reverse the reaction from PrP(Sc) into PrP(C) is considered valuable, as it would give a key to dissolving the complex molecular events into thermodynamic and kinetic analyses and may also provide a means to prevent the formation of PrP(Sc) from PrP(C) eventually in vivo. Here we show that, by applying pressures at kbar range, the "proteinase K-resistant" fibrils (rHaPrP(res)) prepared from hamster prion protein (rHaPrP [23-231]) by seeding with brain homogenate of scrapie-infected hamster, becomes easily digestible. The result is consistent with the notion that rHaPrP(res) fibrils are dissociated into rHaPrP monomers under pressure and that the formation of PrP(Sc) from PrP(C) is thermodynamically controlled. Moreover, the efficient degradation of prion fibrils under pressure provides a novel means of eliminating infectious PrP(Sc) from various systems of pathogenic concern.
Keywords: AFM atomic force microscopy; PK proteinase K; PrPC cellular form of prion protein; PrPSc scrapie form of prion protein; QUIC quaking-induced conversion; Recombinant Hamster prion protein; Western blotting; dissociation of prion fibrils; enzymatic degradation of prion fibrils; pressure-assisted dissociation; proteinase K-resistant prion fibrils; rHaPrP recombinant Hamster prion protein; rHaPrPres PK-resistant recombinant Hamster prion protein.
Figures

Similar articles
-
Modulation of proteinase K-resistant prion protein in cells and infectious brain homogenate by redox iron: implications for prion replication and disease pathogenesis.Mol Biol Cell. 2007 Sep;18(9):3302-12. doi: 10.1091/mbc.e07-04-0317. Epub 2007 Jun 13. Mol Biol Cell. 2007. PMID: 17567949 Free PMC article.
-
Quaternary Structure Changes for PrPSc Predate PrPC Downregulation and Neuronal Death During Progression of Experimental Scrapie Disease.Mol Neurobiol. 2021 Jan;58(1):375-390. doi: 10.1007/s12035-020-02112-z. Epub 2020 Sep 21. Mol Neurobiol. 2021. PMID: 32959170 Free PMC article.
-
Protein Misfolding Cyclic Amplification Cross-Species Products of Mouse-Adapted Scrapie Strain 139A and Hamster-Adapted Scrapie Strain 263K with Brain and Muscle Tissues of Opposite Animals Generate Infectious Prions.Mol Neurobiol. 2017 Jul;54(5):3771-3782. doi: 10.1007/s12035-016-9945-8. Epub 2016 Jun 4. Mol Neurobiol. 2017. PMID: 27259989
-
Prion diseases and emerging prion diseases.Curr Med Chem. 2008;15(9):912-6. doi: 10.2174/092986708783955437. Curr Med Chem. 2008. PMID: 18473798 Review.
-
Proteinase K and the structure of PrPSc: The good, the bad and the ugly.Virus Res. 2015 Sep 2;207:120-6. doi: 10.1016/j.virusres.2015.03.008. Epub 2015 Mar 24. Virus Res. 2015. PMID: 25816779 Review.
Cited by
-
Proteins in Wonderland: The Magical World of Pressure.Biology (Basel). 2021 Dec 21;11(1):6. doi: 10.3390/biology11010006. Biology (Basel). 2021. PMID: 35053003 Free PMC article.
-
Real-time nuclear magnetic resonance spectroscopy in the study of biomolecular kinetics and dynamics.Magn Reson (Gott). 2021 May 11;2(1):291-320. doi: 10.5194/mr-2-291-2021. eCollection 2021. Magn Reson (Gott). 2021. PMID: 37904763 Free PMC article. Review.
References
-
- Prusiner SB. Prions. Proc Natl Acad Sci U S A 1998; 95:13363-83; PMID:9811807; http://dx.doi.org/10.1073/pnas.95.23.13363 - DOI - PMC - PubMed
-
- Bocharova OV, Makarava N, Breydo L, Anderson M, Salnikov VV, Baskakov IV. Annealing PrP fibrils at high temperature results in extension of a proteinase K resistant core. J Biol Chem 2006; 281:2373-9; PMID:16314415; http://dx.doi.org/10.1074/jbc.M510840200 - DOI - PubMed
-
- Race R, Jenny A, Sutton D. Scrapie infectivity and proteinase K-resistant prion protein in sheep placenta, brain, spleen, and lymph node: implications for transmission and antemortem diagnosis. J Infect Dis 1998; 178:949-53; PMID:9806020; http://dx.doi.org/10.1086/515669 - DOI - PubMed
-
- Akasaka K. Probing conformational fluctuation of proteins by pressure perturbation. Chem Rev 2006; 106:1814-35; PMID:16683756; http://dx.doi.org/10.1021/cr040440z - DOI - PubMed
-
- Silva JL, Weber G. Pressure stability of proteins. Annu Rev Phys Chem 1993; 44:89-113; PMID:8257561; http://dx.doi.org/10.1146/annurev.pc.44.100193.000513 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
