Sensitive detection of aggregated prion protein via proximity ligation
- PMID: 25482604
- PMCID: PMC4601298
- DOI: 10.4161/pri.32231
Sensitive detection of aggregated prion protein via proximity ligation
Abstract
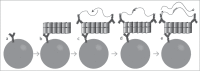
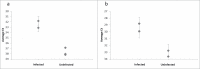
The DNA assisted solid-phase proximity ligation assay (SP-PLA) provides a unique opportunity to specifically detect prion protein (PrP) aggregates by investigating the collocation of 3 or more copies of the specific protein. We have developed an SP-PLA that can detect PrP aggregates in brain homogenates from infected hamsters even after a 10(7)-fold dilution. In contrast, brain homogenate from uninfected animals did not generate a detectable signal at 100-fold higher concentration. Using either of the 2 monoclonal anti-PrP antibodies, 3F4 and 6H4, we successfully detected low concentrations of aggregated PrP. The presented results provide a proof of concept that this method might be an interesting tool in the development of diagnostic approaches of prion diseases.
Keywords: 263K; BSE, bovine spongiform encephalopathy; CJD, Creutzfeldt-Jakob disease; CSF, cerebrospinal fluid; FIDA, fluorescence intensity distribution analysis; PLA, proximity ligation assay; PMCA, protein misfolding cyclic amplification; PrP, prion protein; PrPC, cellular prion protein; PrPSc, scrapie prion protein; QuIC, quaking-induced conversion; SP-PLA, solid phase proximity ligation assay; diagnosis; monoclonal antibody; prion protein; proximity ligation assay; qPCR, quantitative real-time PCR.
Figures


Similar articles
-
Characterisation of new monoclonal antibodies reacting with prions from both human and animal brain tissues.J Immunol Methods. 2008 Sep 15;337(2):106-20. doi: 10.1016/j.jim.2008.07.004. Epub 2008 Jul 25. J Immunol Methods. 2008. PMID: 18657541
-
Human variant Creutzfeldt-Jakob disease and sheep scrapie PrP(res) detection using seeded conversion of recombinant prion protein.Protein Eng Des Sel. 2009 Aug;22(8):515-21. doi: 10.1093/protein/gzp031. Epub 2009 Jul 1. Protein Eng Des Sel. 2009. PMID: 19570812 Free PMC article.
-
Sensitive and specific detection of sporadic Creutzfeldt-Jakob disease brain prion protein using real-time quaking-induced conversion.J Gen Virol. 2012 Feb;93(Pt 2):438-449. doi: 10.1099/vir.0.033365-0. Epub 2011 Oct 26. J Gen Virol. 2012. PMID: 22031526 Free PMC article.
-
Molecular biology of prions causing infectious and genetic encephalopathies of humans as well as scrapie of sheep and BSE of cattle.Dev Biol Stand. 1991;75:55-74. Dev Biol Stand. 1991. PMID: 1686599 Review.
-
Prion encephalopathies of animals and humans.Dev Biol Stand. 1993;80:31-44. Dev Biol Stand. 1993. PMID: 8270114 Review.
Cited by
-
Proximity ligation assay: an ultrasensitive method for protein quantification and its applications in pathogen detection.Appl Microbiol Biotechnol. 2021 Feb;105(3):923-935. doi: 10.1007/s00253-020-11049-1. Epub 2021 Jan 11. Appl Microbiol Biotechnol. 2021. PMID: 33427935 Review.
-
Accurate detection of Newcastle disease virus using proximity-dependent DNA aptamer ligation assays.FEBS Open Bio. 2021 Apr;11(4):1122-1131. doi: 10.1002/2211-5463.13117. Epub 2021 Mar 11. FEBS Open Bio. 2021. PMID: 33595202 Free PMC article.
-
Homogeneous and digital proximity ligation assays for the detection of Clostridium difficile toxins A and B.Biomol Detect Quantif. 2016 Aug 31;10:2-8. doi: 10.1016/j.bdq.2016.06.003. eCollection 2016 Dec. Biomol Detect Quantif. 2016. PMID: 27990343 Free PMC article.
References
-
- Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science 1982; 216:136-44; PMID:6801762; http://dx.doi.org/10.1126/science.6801762 - DOI - PubMed
-
- Gofflot S, Deprez M, Moualij B el, Osman A, Thonnart J-F, Hougrand O, Heinen E, Zorzi W. Immunoquantitative PCR for prion protein detection in sporadic creutzfeldt–jakob disease. Clin Chem 2005; 51:1605-11; PMID:16002456; http://dx.doi.org/10.1373/clinchem.2005.050120 - DOI - PubMed
-
- Barletta JM, Edelman DC, Highsmith WE, Constantine NT. Detection of ultra-low levels of pathologic prion protein in scrapie infected hamster brain homogenates using real-time immuno-PCR. J Virol Methods 2005; 127:154-64; PMID:15921765; http://dx.doi.org/10.1016/j.jviromet.2005.04.007 - DOI - PubMed
-
- Tzaban S, Friedlander G, Schonberger O, Horonchik L, Yedidia Y, Shaked G, Gabizon R, Taraboulos A. Protease-sensitive scrapie prion protein in aggregates of heterogeneous sizes. Biochemistry 2002; 41:12868-75; PMID:12379130; http://dx.doi.org/10.1021/bi025958g - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
