Reprogramming during epithelial to mesenchymal transition under the control of TGFβ
- PMID: 25482613
- PMCID: PMC4594534
- DOI: 10.4161/19336918.2014.983794
Reprogramming during epithelial to mesenchymal transition under the control of TGFβ
Abstract
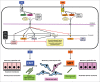
Epithelial-mesenchymal transition (EMT) refers to plastic changes in epithelial tissue architecture. Breast cancer stromal cells provide secreted molecules, such as transforming growth factor β (TGFβ), that promote EMT on tumor cells to facilitate breast cancer cell invasion, stemness and metastasis. TGFβ signaling is considered to be abnormal in the context of cancer development; however, TGFβ acting on breast cancer EMT resembles physiological signaling during embryonic development, when EMT generates or patterns new tissues. Interestingly, while EMT promotes metastatic fate, successful metastatic colonization seems to require the inverse process of mesenchymal-epithelial transition (MET). EMT and MET are interconnected in a time-dependent and tissue context-dependent manner and are coordinated by TGFβ, other extracellular proteins, intracellular signaling cascades, non-coding RNAs and chromatin-based molecular alterations. Research on breast cancer EMT/MET aims at delivering biomolecules that can be used diagnostically in cancer pathology and possibly provide ideas for how to improve breast cancer therapy.
Keywords: BMP, bone morphogenetic protein; CSC, cancer stem cell; DNMT, DNA methyltransferase; EMT, epithelial-mesenchymal transition; FGF, fibroblast growth factor; HDAC, histone deacetylase; MAPK, mitogen activated protein kinase; MET, mesenchymal-epithelial transition; PDGF, platelet derived growth factor; PRC, polycomb repressive complex; TF, transcription factor; TGFβ; bHLH, basic helix-loop-helix; epithelial-mesenchymal transition; lncRNA, long non-coding RNA; mTORC, mammalian target of rapamycin complex; miRNA, micro-RNA; signal transduction; transforming growth factor β; transforming growth factor β.; tumor invasiveness.
Figures
References
-
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell 2009; 139(5):871-90; PMID:19945376; http://dx.doi.org/ 10.1016/j.cell.2009.11.007 - DOI - PubMed
-
- Bissell MJ, Rizki A, Mian IS. Tissue architecture: the ultimate regulator of breast epithelial function. Curr Opin Cell Biol 2003; 15(6):753-62; PMID:14644202; http://dx.doi.org/ 10.1016/j.ceb.2003.10.016 - DOI - PMC - PubMed
-
- Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat (Basel) 1995; 154(1):8-20; PMID:8714286; http://dx.doi.org/ 10.1159/000147748 - DOI - PubMed
-
- Ansieau S, Bastid J, Doreau A, Morel AP, Bouchet BP, Thomas C, Fauvet F, Puisieux I, Doglioni C, Piccinin S, et al. Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell 2008; 14(1):79-89; PMID:18598946; http://dx.doi.org/ 10.1016/j.ccr.2008.06.005 - DOI - PubMed
-
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008; 133(4):704-15; PMID:18485877; http://dx.doi.org/ 10.1016/j.cell.2008.03.027 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
