Cadherins as regulators of neuronal polarity
- PMID: 25482615
- PMCID: PMC4594569
- DOI: 10.4161/19336918.2014.983808
Cadherins as regulators of neuronal polarity
Abstract
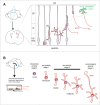
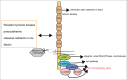
A compelling amount of data is accumulating about the polyphonic role of neuronal cadherins during brain development throughout all developmental stages, starting from the involvement of cadherins in the organization of neurulation up to synapse development and plasticity. Recent work has confirmed that specifically N-cadherins play an important role in asymmetrical cellular processes in developing neurons that are at the basis of polarity. In this review we will summarize recent data, which demonstrate how N-cadherin orchestrates distinct processes of polarity establishment in neurons.
Keywords: N-cadherin; adhesion; axon; migration; neuronal polarity.
Figures

References
-
- Gleeson JG. Neuronal migration disorders. Ment Retard Dev Disabil Res Rev 2001; 7:167-71; PMID:11553932; http://dx.doi.org/10.1002/mrdd.1024 - DOI - PubMed
-
- Gotz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol 2005; 6:777-88; PMID:16314867; http://dx.doi.org/10.1038/nrm1739 - DOI - PubMed
-
- Franco SJ, Muller U. Shaping our minds: stem and progenitor cell diversity in the mammalian neocortex. Neuron 2013; 77:19-34; PMID:23312513; http://dx.doi.org/10.1016/j.neuron.2012.12.022 - DOI - PMC - PubMed
-
- Paridaen JT, Huttner WB. Neurogenesis during development of the vertebrate central nervous system. EMBO Rep 2014; 15:351-64; PMID:24639559; http://dx.doi.org/10.1002/embr.201438447 - DOI - PMC - PubMed
-
- Angevine JB, Jr., Sidman RL. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature 1961; 192:766-8; PMID:17533671; http://dx.doi.org/10.1038/192766b0 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
