Rsu1 contributes to cell adhesion and spreading in MCF10A cells via effects on P38 map kinase signaling
- PMID: 25482629
- PMCID: PMC4594256
- DOI: 10.4161/19336918.2014.972775
Rsu1 contributes to cell adhesion and spreading in MCF10A cells via effects on P38 map kinase signaling
Abstract
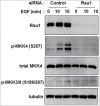
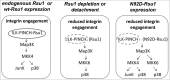
The ILK, PINCH, Parvin (IPP) complex regulates adhesion and migration via binding of ILK to β1 integrin and α-parvin thus linking focal adhesions to actin cytoskeleton. ILK also binds the adaptor protein PINCH which connects signaling proteins including Rsu1 to the complex. A recent study of Rsu1 and PINCH1 in non-transformed MCF10A human mammary epithelial cells revealed that the siRNA-mediated depletion of either Rsu1 or PINCH1 decreased the number of focal adhesions (FAs) and altered the distribution and localization of FA proteins. This correlated with reduced adhesion, failure to spread or migrate in response to EGF and a loss of actin stress fibers and caveolae. The depletion of Rsu1 caused significant reduction in PINCH1 implying that Rsu1 may function in part by regulating levels of PINCH1. However, Rsu1, but not PINCH1, was required for EGF-induced activation of p38 Map kinase and ATF2 phosphorylation, suggesting a Rsu1 function independent from the IPP complex. Reconstitution of Rsu1-depleted cells with a Rsu1 mutant (N92D) that does not bind to PINCH1 failed to restore FAs or migration but did promote IPP-independent spreading and constitutive as well as EGF-induced p38 activation. In this commentary we discuss p38 activity in adhesion and how Rsu1 expression may be linked to Map kinase kinase (MKK) activation and detachment-induced stress kinase signaling.
Keywords: FA; focal adhesion; FAK; focal adhesion kinase; ILK; Integrin linked kinase; IPP; Intergrin linked kinase-PINCH-Parvin; LRR; leucine rich repeat; MCF10A cells; MKK; Map kinase kinase; NLS; nuclear localization sequence; PINCH1; RALGEF; Ral Guanine nucleotide exchange factor; ROS; reactive oxygen species; Rsu1; Rsu1; Ras suppressor protein 1.; adhesion; migration; p38 Map kinase.
Figures
References
-
- Cabodi S, del Pilar Camacho-Leal M, Di Stefano P, Defilippi P. Integrin signalling adaptors: not only figurants in the cancer story. Nat Rev Cancer 2010; 10:858-70; PMID:21102636; http://dx.doi.org/10.1038/nrc2967 - DOI - PubMed
-
- Qin J, Wu C. ILK: a pseudokinase in the center stage of cell-matrix adhesion and signaling. Curr Opin Cell Biol 2012; 24:607-13; PMID:22763012; http://dx.doi.org/10.1016/j.ceb.2012.06.003 - DOI - PMC - PubMed
-
- Stanchi F, Grashoff C, Nguemeni Yonga CF, Grall D, Fassler R, Van Obberghen-Schilling E. Molecular dissection of the ILK-PINCH-parvin triad reveals a fundamental role for the ILK kinase domain in the late stages of focal-adhesion maturation. J Cell Sci 2009; 122:1800-11. http://jcs.biologists.org/content/122/11/1800.full.pdf; http://dx.doi.org/10.1242/jcs.044602 - DOI - PubMed
-
- Wickstrom SA, Lange A, Montanez E, Fassler R. The ILK/PINCH/parvin complex: the kinase is dead, long live the pseudokinase! EMBO J 2010; 29:281-91. http://www.nature.com/emboj /journal /v29/n2/pdf/emboj2009376a.pdf; http://dx.doi.org/10.1038/emboj.2009.376 - DOI - PMC - PubMed
-
- Tu Y, Huang Y, Zhang Y, Hua Y, Wu C. A new focal adhesion protein that interacts with integrin-linked kinase and regulates cell adhesion and spreading. J Cell Biol 2001; 153:585-98. http://pubmedcentralcanada.ca/picrender.cgi?accid=PMC2190577&blobtype=pdf; http://dx.doi.org/10.1083/jcb.153.3.585 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
