Nck adaptors, besides promoting N-WASP mediated actin-nucleation activity at pedestals, influence the cellular levels of enteropathogenic Escherichia coli Tir effector
- PMID: 25482634
- PMCID: PMC4594261
- DOI: 10.4161/19336918.2014.969993
Nck adaptors, besides promoting N-WASP mediated actin-nucleation activity at pedestals, influence the cellular levels of enteropathogenic Escherichia coli Tir effector
Abstract
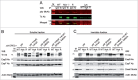
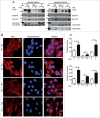
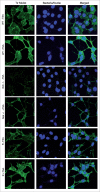
Enteropathogenic Escherichia coli (EPEC) binding to human intestinal cells triggers the formation of disease-associated actin rich structures called pedestals. The latter process requires the delivery, via a Type 3 secretion system, of the translocated Intimin receptor (Tir) protein into the host plasma membrane where binding of a host kinase-modified form to the bacterial surface protein Intimin triggers pedestal formation. Tir-Intimin interaction recruits the Nck adaptor to a Tir tyrosine phosphorylated residue where it activates neural Wiskott-Aldrich syndrome protein (N-WASP); initiating the major pathway to actin polymerization mediated by the actin-related protein (Arp) 2/3 complex. Previous studies with Nck-deficient mouse embryonic fibroblasts (MEFs) identified a key role for Nck in pedestal formation, presumed to reflect a lack of N-WASP activation. Here, we show the defect relates to reduced amounts of Tir within Nck-deficient cells. Indeed, Tir delivery and, thus, pedestal formation defects were much greater for MEFs than HeLa (human epithelial) cells. Crucially, the levels of two other effectors (EspB/EspF) within Nck-deficient MEFs were not reduced unlike that of Map (Mitochondrial associated protein) which, like Tir, requires CesT chaperone function for efficient delivery. Interestingly, drugs blocking various host protein degradation pathways failed to increase Tir cellular levels unlike an inhibitor of deacetylase activity (Trichostatin A; TSA). Treatments with TSA resulted in significant recovery of Tir levels, potentiation of actin polymerization and improvement in bacterial attachment to cells. Our findings have important implications for the current model of Tir-mediated actin polymerization and opens new lines of research in this area.
Keywords: A/E, Attaching and effacing; Ab, Polyclonal antibody; Actin; BFP, Bundle-forming pili; Cmp, Chloramphenicol; CrkII, CT10 regulator of kinase; CrkL, Crk-like; Ctr., Control; EHEC, Enterohaemorrhagic Escherichia coli; EPEC; EPEC, Enteropathogenic Escherichia coli; Esp, EPEC-secreted proteins; FBS, Fetal bovine serum; GBD, GTPase-binding domain; HDAC, Histone deacetylases; HeLa, Human cervical epithelial cancer cell line; IRSp53, Insulin receptor tyrosine kinase substrate p53; IRTKS, Insulin receptor tyrosine kinase substrate; LEE, Locus of enterocyte effacement; MEFs, Mouse embryonic fibroblasts; MOI, Multiplicity of infection; Map, Mitochondrial associated protein; MoAb, Monoclonal antibody; N-WASP; N-WASP, Neural Wiskott–Aldrich syndrome protein; NF-kB, Nuclear factor kB; NPF, Nucleation promoting factor; Nck; Nck, Non-catalytic tyrosine kinase; Nle, Non-LEE effectors; PRD, Proline-rich domain; SH3, Src homology 3; T3SS, Type 3 secretion system; TNF- α, Tumor necrosis factor-α; TSA; TSA, Trichostatin A; Tir; Tir, Translocated Intimin receptor; WB, Western Blot; WT, Wild type.; bacterial adhesion; pedestals.
Figures




Similar articles
-
Tir phosphorylation and Nck/N-WASP recruitment by enteropathogenic and enterohaemorrhagic Escherichia coli during ex vivo colonization of human intestinal mucosa is different to cell culture models.Cell Microbiol. 2007 May;9(5):1352-64. doi: 10.1111/j.1462-5822.2006.00879.x. Cell Microbiol. 2007. PMID: 17474908
-
Crk adaptors negatively regulate actin polymerization in pedestals formed by enteropathogenic Escherichia coli (EPEC) by binding to Tir effector.PLoS Pathog. 2014 Mar 27;10(3):e1004022. doi: 10.1371/journal.ppat.1004022. eCollection 2014 Mar. PLoS Pathog. 2014. PMID: 24675776 Free PMC article.
-
Enteropathogenic E. coli Tir binds Nck to initiate actin pedestal formation in host cells.Nat Cell Biol. 2001 Sep;3(9):856-9. doi: 10.1038/ncb0901-856. Nat Cell Biol. 2001. PMID: 11533668
-
Attaching effacing Escherichia coli and paradigms of Tir-triggered actin polymerization: getting off the pedestal.Cell Microbiol. 2008 Mar;10(3):549-56. doi: 10.1111/j.1462-5822.2007.01103.x. Epub 2007 Dec 4. Cell Microbiol. 2008. PMID: 18053003 Review.
-
Tails of two Tirs: actin pedestal formation by enteropathogenic E. coli and enterohemorrhagic E. coli O157:H7.Curr Opin Microbiol. 2003 Feb;6(1):82-90. doi: 10.1016/s1369-5274(03)00005-5. Curr Opin Microbiol. 2003. PMID: 12615225 Review.
Cited by
-
Plasticity of the brush border - the yin and yang of intestinal homeostasis.Nat Rev Gastroenterol Hepatol. 2016 Mar;13(3):161-74. doi: 10.1038/nrgastro.2016.5. Epub 2016 Feb 3. Nat Rev Gastroenterol Hepatol. 2016. PMID: 26837713 Review.
-
BAIAP2 as a driver of tumor progression in urothelial bladder cancer.BMC Cancer. 2025 Jul 1;25(1):1057. doi: 10.1186/s12885-025-14470-9. BMC Cancer. 2025. PMID: 40596964 Free PMC article.
-
β-arrestin 2 quenches TLR signaling to facilitate the immune evasion of EPEC.Gut Microbes. 2020 Sep 2;11(5):1423-1437. doi: 10.1080/19490976.2020.1759490. Epub 2020 May 13. Gut Microbes. 2020. PMID: 32403971 Free PMC article.
-
GWAS analysis of QTL for enteric septicemia of catfish and their involved genes suggest evolutionary conservation of a molecular mechanism of disease resistance.Mol Genet Genomics. 2017 Feb;292(1):231-242. doi: 10.1007/s00438-016-1269-x. Epub 2016 Nov 8. Mol Genet Genomics. 2017. PMID: 27826737
-
Proteogenomic discovery of sORF-encoded peptides associated with bacterial virulence in Yersinia pestis.Commun Biol. 2021 Nov 2;4(1):1248. doi: 10.1038/s42003-021-02759-x. Commun Biol. 2021. PMID: 34728737 Free PMC article.
References
-
- Navarro-Garcia F, Serapio-Palacios A, Ugalde-Silva P, Tapia-Pastrana G, Chavez-Duenas L. Actin cytoskeleton manipulation by effector proteins secreted by diarrheagenic Escherichia coli pathotypes. Biomed Res Int 2013; 2013:374395; PMID:23509714; http://dx.doi.org/10.1155/2013/374395 - DOI - PMC - PubMed
-
- Donnenberg MS, Tacket CO, James SP, Losonsky G, Nataro JP, Wasserman SS, Wasserman SS, Kaper JB, Levine MM. Role of the eaeA gene in experimental enteropathogenic Escherichia coli infection. J Clin Invest 1993; 92:1412-7; PMID:8376594; http://dx.doi.org/10.1172/JCI116717 - DOI - PMC - PubMed
-
- Marches O, Nougayrede JP, Boullier S, Mainil J, Charlier G, Raymond I, Pohl P, Boury M, De Rycke J, Milon A, et al. . Role of tir and intimin in the virulence of rabbit enteropathogenic Escherichia coli serotype O103:H2. Infect Immun 2000; 68:2171-82; PMID:10722617; http://dx.doi.org/10.1128/IAI.68.4.2171-2182.2000 - DOI - PMC - PubMed
-
- Giron JA, Ho AS, Schoolnik GK. An inducible bundle-forming pilus of enteropathogenic Escherichia coli. Science 1991; 254:710-3; PMID:1683004; http://dx.doi.org/10.1126/science.1683004 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
