Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial)
- PMID: 25482832
- PMCID: PMC4407930
- DOI: 10.1002/hep.27647
Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial)
Abstract
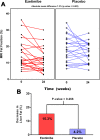
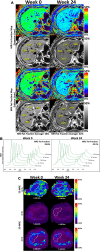
Ezetimibe inhibits intestinal cholesterol absorption and lowers low-density lipoprotein cholesterol. Uncontrolled studies have suggested that it reduces liver fat as estimated by ultrasound in nonalcoholic steatohepatitis (NASH). Therefore, we aimed to examine the efficacy of ezetimibe versus placebo in reducing liver fat by the magnetic resonance imaging-derived proton density-fat fraction (MRI-PDFF) and liver histology in patients with biopsy-proven NASH. In this randomized, double-blind, placebo-controlled trial, 50 patients with biopsy-proven NASH were randomized to either ezetimibe 10 mg orally daily or placebo for 24 weeks. The primary outcome was a change in liver fat as measured by MRI-PDFF in colocalized regions of interest within each of the nine liver segments. Novel assessment by two-dimensional and three-dimensional magnetic resonance elastography was also performed. Ezetimibe was not significantly better than placebo at reducing liver fat as measured by MRI-PDFF (mean difference between the ezetimibe and placebo arms -1.3%, P = 0.4). Compared to baseline, however, end-of-treatment MRI-PDFF was significantly lower in the ezetimibe arm (15%-11.6%, P < 0.016) but not in the placebo arm (18.5%-16.4%, P = 0.15). There were no significant differences in histologic response rates, serum alanine aminotransferase and aspartate aminotransferase levels, or longitudinal changes in two-dimensional and three-dimensional magnetic resonance elastography-derived liver stiffness between the ezetimibe and placebo arms. Compared to histologic nonresponders (25/35), histologic responders (10/35) had a significantly greater reduction in MRI-PDFF (-4.35 ± 4.9% versus -0.30 ± 4.1%, P < 0.019).
Conclusions: Ezetimibe did not significantly reduce liver fat in NASH. This trial demonstrates the application of colocalization of MRI-PDFF-derived fat maps and magnetic resonance elastography-derived stiffness maps of the liver before and after treatment to noninvasively assess treatment response in NASH.
© 2014 The Authors. HEPATOLOGY published by Wiley Periodicals, Inc., on behalf of the American Association for the Study of Liver Diseases.
Figures
Similar articles
-
Sitagliptin vs. placebo for non-alcoholic fatty liver disease: A randomized controlled trial.J Hepatol. 2016 Aug;65(2):369-76. doi: 10.1016/j.jhep.2016.04.021. Epub 2016 May 2. J Hepatol. 2016. PMID: 27151177 Free PMC article. Clinical Trial.
-
Assessment of treatment response in non-alcoholic steatohepatitis using advanced magnetic resonance imaging.Aliment Pharmacol Ther. 2017 Mar;45(6):844-854. doi: 10.1111/apt.13951. Epub 2017 Jan 24. Aliment Pharmacol Ther. 2017. PMID: 28116801 Free PMC article. Clinical Trial.
-
Association of noninvasive quantitative decline in liver fat content on MRI with histologic response in nonalcoholic steatohepatitis.Therap Adv Gastroenterol. 2016 Sep;9(5):692-701. doi: 10.1177/1756283X16656735. Epub 2016 Jul 5. Therap Adv Gastroenterol. 2016. PMID: 27582882 Free PMC article.
-
Change in MRI-PDFF and Histologic Response in Patients With Nonalcoholic Steatohepatitis: A Systematic Review and Meta-Analysis.Clin Gastroenterol Hepatol. 2021 Nov;19(11):2274-2283.e5. doi: 10.1016/j.cgh.2020.08.061. Epub 2020 Aug 31. Clin Gastroenterol Hepatol. 2021. PMID: 32882428 Free PMC article.
-
Rates of and Factors Associated With Placebo Response in Trials of Pharmacotherapies for Nonalcoholic Steatohepatitis: Systematic Review and Meta-analysis.Clin Gastroenterol Hepatol. 2019 Mar;17(4):616-629.e26. doi: 10.1016/j.cgh.2018.06.011. Epub 2018 Jun 18. Clin Gastroenterol Hepatol. 2019. PMID: 29913275
Cited by
-
The impact of genetic risk on liver fibrosis in non-alcoholic fatty liver disease as assessed by magnetic resonance elastography.Aliment Pharmacol Ther. 2021 Jul;54(1):68-77. doi: 10.1111/apt.16392. Epub 2021 May 11. Aliment Pharmacol Ther. 2021. PMID: 33975381 Free PMC article.
-
Racial disparities in nonalcoholic fatty liver disease clinical trial enrollment: A systematic review and meta-analysis.World J Hepatol. 2020 Aug 27;12(8):506-518. doi: 10.4254/wjh.v12.i8.506. World J Hepatol. 2020. PMID: 32952877 Free PMC article.
-
Magnetic Resonance Elastography of Liver: Current Update.Top Magn Reson Imaging. 2018 Oct;27(5):319-333. doi: 10.1097/RMR.0000000000000177. Top Magn Reson Imaging. 2018. PMID: 30289828 Free PMC article. Review.
-
Ezetimibe combination therapy with statin for non-alcoholic fatty liver disease: an open-label randomized controlled trial (ESSENTIAL study).BMC Med. 2022 Mar 21;20(1):93. doi: 10.1186/s12916-022-02288-2. BMC Med. 2022. PMID: 35307033 Free PMC article. Clinical Trial.
-
Prospective, Same-Day, Direct Comparison of Controlled Attenuation Parameter With the M vs the XL Probe in Patients With Nonalcoholic Fatty Liver Disease, Using Magnetic Resonance Imaging-Proton Density Fat Fraction as the Standard.Clin Gastroenterol Hepatol. 2020 Jul;18(8):1842-1850.e6. doi: 10.1016/j.cgh.2019.11.060. Epub 2019 Dec 13. Clin Gastroenterol Hepatol. 2020. PMID: 31843596 Free PMC article.
References
-
- Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. et al. - PubMed
-
- Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131. et al. - PubMed
-
- Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. et al. - PubMed
-
- Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver versus nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. doi: 10.1016/j.cgh.2014.04.014. - DOI - PMC - PubMed
-
- Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249–1253. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
