The left-right Pitx2 pathway drives organ-specific arterial and lymphatic development in the intestine
- PMID: 25482882
- PMCID: PMC4326534
- DOI: 10.1016/j.devcel.2014.11.002
The left-right Pitx2 pathway drives organ-specific arterial and lymphatic development in the intestine
Abstract
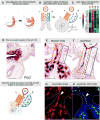
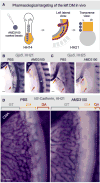
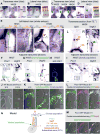
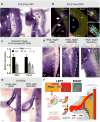
The dorsal mesentery (DM) is the major conduit for blood and lymphatic vessels in the gut. The mechanisms underlying their morphogenesis are challenging to study and remain unknown. Here we show that arteriogenesis in the DM begins during gut rotation and proceeds strictly on the left side, dependent on the Pitx2 target gene Cxcl12. Although competent Cxcr4-positive angioblasts are present on the right, they fail to form vessels and progressively emigrate. Surprisingly, gut lymphatics also initiate in the left DM and arise only after-and dependent on-arteriogenesis, implicating arteries as drivers of gut lymphangiogenesis. Our data begin to unravel the origin of two distinct vascular systems and demonstrate how early left-right molecular asymmetries are translated into organ-specific vascular patterns. We propose a dual origin of gut lymphangiogenesis in which prior arterial growth is required to initiate local lymphatics that only subsequently connect to the vascular system.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures




References
-
- Alexander JS, Chaitanya GV, Grisham MB, Boktor M. Emerging roles of lymphatics in inflammatory bowel disease. Annals of the New York Academy of Sciences. 2010;1207(Suppl 1):E75–85. - PubMed
-
- Ara T, Tokoyoda K, Okamoto R, Koni PA, Nagasawa T. The role of CXCL12 in the organ-specific process of artery formation. Blood. 2005;105:3155–3161. - PubMed
-
- Aselli G. De lactibus, sive Lacteis venis, quarto vasorum mesaraicorum genere novo invento dissertatio. Medioloni, Milan; 1627.
-
- Betterman KL, Paquet-Fifield S, Asselin-Labat ML, Visvader JE, Butler LM, Stacker SA, Achen MG, Harvey NL. Remodeling of the lymphatic vasculature during mouse mammary gland morphogenesis is mediated via epithelial-derived lymphangiogenic stimuli. The American journal of pathology. 2012;181:2225–2238. - PubMed
-
- Bulow HE, Hobert O. The molecular diversity of glycosaminoglycans shapes animal development. Annu Rev Cell Dev Biol. 2006;22:375–407. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

