Dasatinib reverses the multidrug resistance of breast cancer MCF-7 cells to doxorubicin by downregulating P-gp expression via inhibiting the activation of ERK signaling pathway
- PMID: 25482933
- PMCID: PMC4622436
- DOI: 10.4161/15384047.2014.987062
Dasatinib reverses the multidrug resistance of breast cancer MCF-7 cells to doxorubicin by downregulating P-gp expression via inhibiting the activation of ERK signaling pathway
Abstract
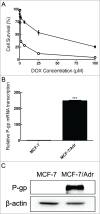
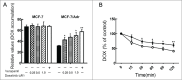
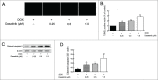
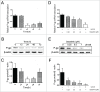
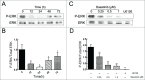
Multidrug resistance (MDR) is one of the major obstacles to the efficiency of cancer chemotherapy, which often results from the overexpression of drug efflux transporters such as P-glycoprotein (P-gp). In the present study, we determined the effect of dasatinib which was approved for imatinib resistant chronic myelogenous leukemia (CML) and (Ph(+)) acute lymphoblastic leukemia (ALL) treatment on P-gp-mediated MDR. Our results showed that dasatinib significantly increased the sensitivity of P-gp-overexpressing MCF-7/Adr cells to doxorubicin in MTT assays; thus lead to an enhanced cytotoxicity of doxorubicin in MCF-7/Adr cells. Additionally, dasatinib increased the intracellular accumulation, inhibited the efflux of doxorubicin in MCF-7/Adr cells, and significantly enhanced doxorubicin-induced apoptosis in MCF-7/Adr cells. Further studies showed that dasatinib altered the expression levels of mRNA, protein levels of P-gp, and the phosphorylation of signal-regulated kinase (ERK) both in time-dependent (before 24 h) and dose-dependent manners at concentrations that produced MDR reversals. In conclusion, dasatinib reverses P-gp-mediated MDR by downregulating P-gp expression, which may be partly attributed to the inhibition of ERK pathway. Dasatinib may play an important role in circumventing MDR when combined with other conventional antineoplastic drugs.
Keywords: DOX, doxorubicin; ERK pathway; ERKextracellular signal-regulated kinase; MDR, multidrug resistance; P-ERK, phosphorylated extracellular signal–regulated kinase; P-glycoprotein; P-gp, P-glycoprotein; dasatinib; doxorubicin; multidrug resistance.
Figures
References
-
- Litman T, Druley TE, Stein WD, Bates SE. From MDR to MXR: new understanding of multidrug resistance systems, their properties and clinical significance. Cell Mol Life Sci 2001; 58: 931-59; PMID:11497241; http://dx.doi.org/10.1007/PL00000912 - DOI - PMC - PubMed
-
- Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta 1976; 455: 152-62; PMID:990323; http://dx.doi.org/10.1016/0005-2736(76)90160-7 - DOI - PubMed
-
- Ambudkar SV, Kimchi-Sarfaty C, Sauna ZE, Gottesman MM. P-glycoprotein: from genomics to mechanism. Oncogene 2003; 22: 7468-85; PMID:14576852; http://dx.doi.org/10.1038/sj.onc.1206948 - DOI - PubMed
-
- Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer 2002; 2: 48-58; PMID:11902585; http://dx.doi.org/10.1038/nrc706 - DOI - PubMed
-
- Ghayad SE, Vendrell JA, Ben Larbi S, Dumontet C, Bieche I, Cohen PA. Endocrine resistance associated with activated ErbB system in breast cancer cells is reversed by inhibiting MAPK or PI3K/Akt signaling pathways. Int J Cancer 2010; 126: 545-62; PMID:19609946; http://dx.doi.org/10.1002/ijc.24750 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
