Increasing cisplatin sensitivity by schedule-dependent inhibition of AKT and Chk1
- PMID: 25482935
- PMCID: PMC4623033
- DOI: 10.4161/15384047.2014.961876
Increasing cisplatin sensitivity by schedule-dependent inhibition of AKT and Chk1
Abstract
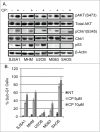
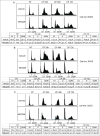
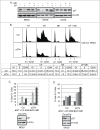
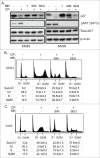
The effectiveness of DNA damaging chemotherapy drugs can be limited by activation of survival signaling pathways and cell cycle checkpoints that allow DNA repair. Targeting survival pathways and inhibiting cell cycle checkpoints may increase chemotherapy-induced cancer cell killing. AKT and Chk1 are survival and cell cycle checkpoint kinases, respectively, that can be activated by DNA damage. Cisplatin (CP) is a standard chemotherapy agent for osteosarcoma (OS). CP induced apoptosis to varying extents and activated AKT and Chk1 in multiple p53 wild-type and p53-null OS cell lines. A Chk1 inhibitor increased CP-induced apoptosis in all OS cell lines regardless of p53 status. In contrast, an AKT inhibitor increased CP-induced apoptosis only in p53 wild-type OS cells, but not p53 nulll cells. The increased apoptosis in p53 wild-type cells was coincident with decreased p53 protein levels, but increased expression of p53-responsive apoptotic genes Noxa and PUMA. Further studies revealed the inability of AKT inhibitor to CP-sensitize p53-null OS cells resulted from 2 things: 1) AKT inhibition stabilized/maintained p27 levels in CP-treated cells, which then mediated a protective G1-phase cell cycle arrest, 2) AKT inhibition increased the levels of activated Chk1. Finally, schedule dependent inhibition of AKT and Chk1 evaded the protective G1 arrest mediated by p27 and maximized CP-induced OS cell killing. These data demonstrate AKT and Chk1 activation promote survival in CP-treated OS cells, and that strategic, scheduled targeting of AKT and Chk1 can maximize OS cell killing by CP.
Keywords: AKT; CDC2, cell division cycle kinase 2; Chk1; Chk1, Checkpoint kinase 1; apoptosis; cisplatin; mTORC1 and mTORC2, mammalian target of rapamycin complex 1 and 2; osteosarcoma; p53.
Figures







Similar articles
-
Crosstalk between the IGF-1R/AKT/mTORC1 pathway and the tumor suppressors p53 and p27 determines cisplatin sensitivity and limits the effectiveness of an IGF-1R pathway inhibitor.Oncotarget. 2016 May 10;7(19):27511-26. doi: 10.18632/oncotarget.8484. Oncotarget. 2016. PMID: 27050276 Free PMC article.
-
Inhibition of AKT sensitizes chemoresistant ovarian cancer cells to cisplatin by abrogating S and G2/M arrest.Exp Mol Pathol. 2016 Jun;100(3):506-13. doi: 10.1016/j.yexmp.2016.05.003. Epub 2016 May 6. Exp Mol Pathol. 2016. PMID: 27163202
-
Knockdown of Chk1 sensitizes human colon carcinoma HCT116 cells in a p53-dependent manner to lidamycin through abrogation of a G2/M checkpoint and induction of apoptosis.Cancer Biol Ther. 2009 Aug;8(16):1559-66. doi: 10.4161/cbt.8.16.8955. Epub 2009 Aug 8. Cancer Biol Ther. 2009. PMID: 19502782
-
Targeting serine/threonine protein kinase B/Akt and cell-cycle checkpoint kinases for treating cancer.Curr Top Med Chem. 2002 Sep;2(9):939-71. doi: 10.2174/1568026023393318. Curr Top Med Chem. 2002. PMID: 12171565 Review.
-
Chk1 inhibitors for novel cancer treatment.Anticancer Agents Med Chem. 2006 Jul;6(4):377-88. doi: 10.2174/187152006777698132. Anticancer Agents Med Chem. 2006. PMID: 16842237 Review.
Cited by
-
2-methylbenzoyl berbamine, a multi-targeted inhibitor, suppresses the growth of human osteosarcoma through disabling NF-κB, ERK and AKT signaling networks.Aging (Albany NY). 2020 Jul 26;12(14):15037-15049. doi: 10.18632/aging.103565. Epub 2020 Jul 26. Aging (Albany NY). 2020. PMID: 32713851 Free PMC article.
-
Chk1 Inhibitor SCH900776 Effectively Potentiates the Cytotoxic Effects of Platinum-Based Chemotherapeutic Drugs in Human Colon Cancer Cells.Neoplasia. 2017 Oct;19(10):830-841. doi: 10.1016/j.neo.2017.08.002. Epub 2017 Sep 6. Neoplasia. 2017. PMID: 28888100 Free PMC article.
-
Inhibitory effects of low-intensity pulsed ultrasound sonication on the proliferation of osteosarcoma cells.Oncol Lett. 2017 Sep;14(3):3071-3076. doi: 10.3892/ol.2017.6472. Epub 2017 Jun 23. Oncol Lett. 2017. PMID: 28928844 Free PMC article.
-
TIMP3 regulates osteosarcoma cell migration, invasion, and chemotherapeutic resistances.Tumour Biol. 2016 Jul;37(7):8857-67. doi: 10.1007/s13277-015-4757-4. Epub 2016 Jan 9. Tumour Biol. 2016. PMID: 26749283
-
Targeting AKT as a promising strategy for SOX2-positive, chemoresistant osteosarcoma.Bone Res. 2025 Feb 24;13(1):25. doi: 10.1038/s41413-024-00395-9. Bone Res. 2025. PMID: 39994220 Free PMC article.
References
-
- Eastman A. Cell cycle checkpoints and their impact on anticancer therapeutic strategies. J Cell Biochem 2004; 91:223-31; PMID:14743382; http://dx.doi.org/10.1002/jcb.10699 - DOI - PubMed
-
- Mitchison TJ. The proliferation rate paradox in antimitotic chemotherapy. Mol Biol Cell 2012; 23:1-6; PMID:22210845; http://dx.doi.org/10.1091/mbc.E10-04-0335 - DOI - PMC - PubMed
-
- Lu C, Fu W, Zhao D, Mattson MP. The DNA damaging agent etoposide activates a cell survival pathway involving alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate receptors and mitogen-activated protein kinases in hippocampal neurons. J Neurosci Res 2002; 70:671-9; PMID:12424735; http://dx.doi.org/10.1002/jnr.10413 - DOI - PubMed
-
- Wang X, McCullough KD, Franke TF, Holbrook NJ. Epidermal growth factor receptor-dependent Akt activation by oxidative stress enhances cell survival. J Biol Chem 2000; 275:14624-31; PMID:10799549; http://dx.doi.org/10.1074/jbc.275.19.14624 - DOI - PubMed
-
- Li HF, Kim JS, Waldman T. Radiation-induced Akt activation modulates radioresistance in human glioblastoma cells. Radiation Oncol 2009; 4:43; http://dx.doi.org/10.1186/1748-717X-4-43 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
