Mitotic catenation is monitored and resolved by a PKCε-regulated pathway
- PMID: 25483024
- PMCID: PMC4272242
- DOI: 10.1038/ncomms6685
Mitotic catenation is monitored and resolved by a PKCε-regulated pathway
Abstract
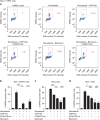
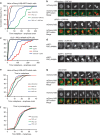
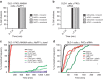
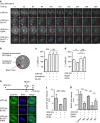
Exit from mitosis is controlled by silencing of the spindle assembly checkpoint (SAC). It is important that preceding exit, all sister chromatid pairs are correctly bioriented, and that residual catenation is resolved, permitting complete sister chromatid separation in the ensuing anaphase. Here we determine that the metaphase response to catenation in mammalian cells operates through PKCε. The PKCε-controlled pathway regulates exit from the SAC only when mitotic cells are challenged by retained catenation and this delayed exit is characterized by BubR1-high and Mad2-low kinetochores. In addition, we show that this pathway is necessary to facilitate resolution of retained catenanes in mitosis. When delayed by catenation in mitosis, inhibition of PKCε results in premature entry into anaphase with PICH-positive strands and chromosome bridging. These findings demonstrate the importance of PKCε-mediated regulation in protection from loss of chromosome integrity in cells failing to resolve catenation in G2.
Figures


References
-
- Uhlmann F., Lottspeich F. & Nasmyth K. Sister-chromatid separation at anaphase onset is promoted by cleavage of the cohesin subunit Scc1. Nature 400, 37–42 (1999). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

