Geroconversion: irreversible step to cellular senescence
- PMID: 25483060
- PMCID: PMC4614001
- DOI: 10.4161/15384101.2014.985507
Geroconversion: irreversible step to cellular senescence
Abstract
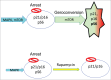
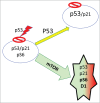
Cellular senescence happens in 2 steps: cell cycle arrest followed, or sometimes preceded, by gerogenic conversion (geroconversion). Geroconvesrion is a form of growth, a futile growth during cell cycle arrest. It converts reversible arrest to irreversible senescence. Geroconversion is driven by growth-promoting, mitogen-/nutrient-sensing pathways such as mTOR. Geroconversion leads to hyper-secretory, hypertrophic and pro-inflammatory cellular phenotypes, hyperfunctions and malfunctions. On organismal level, geroconversion leads to age-related diseases and death. Rapamycin, a gerosuppressant, extends life span in diverse species from yeast to mammals. Stress-and oncogene-induced accelerated senescence, replicative senescence in vitro and life-long cellular aging in vivo all can be described by 2-step model.
Keywords: aging; cell cycle arrest; gerogenic conversion; mTOR; oncogenic trsnformation.
Figures


References
-
- Mathon NF, Lloyd AC. Cell senescence and cancer. Nature Rev Cancer 2001; 1:203-213; http://dx.doi.org/10.1038/35106045 - DOI - PubMed
-
- Dulic V, Beney GE, Frebourg G, Drullinger LF, Stein GH. Uncoupling between phenotypic senescence and cell cycle arrest in aging p21-deficient fibroblasts. Mol Cell Biol 2000; 20:6741-6754; PMID:10958672; http://dx.doi.org/10.1128/MCB.20.18.6741-6754.2000 - DOI - PMC - PubMed
-
- Wang Y, Meng A, Zhou D. Inhibition of phosphatidylinostol 3-kinase uncouples H2O2-induced senescent phenotype and cell cycle arrest in normal human diploid fibroblasts. Exp Cell Res 2004; 298:188-196; PMID:15242773; http://dx.doi.org/10.1016/j.yexcr.2004.04.012 - DOI - PubMed
-
- Campisi J. Suppressing cancer: the importance of being senescent. Science 2005; 309:886-887; PMID:16081723; http://dx.doi.org/10.1126/science.1116801 - DOI - PubMed
-
- Kang TW, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, Dauch D, Hohmeyer A, Gereke M, Rudalska R, Potapova A, et al. . Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 2011; 479:547-551; PMID:22080947; http://dx.doi.org/10.1038/nature10599 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
