Anti-PcrV antibody strategies against virulent Pseudomonas aeruginosa
- PMID: 25483637
- PMCID: PMC5443083
- DOI: 10.4161/21645515.2014.971641
Anti-PcrV antibody strategies against virulent Pseudomonas aeruginosa
Abstract
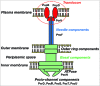
Pseudomonas aeruginosa is an opportunistic bacterial pathogen that causes fatal acute lung infections in critically ill individuals. Its pathogenesis is associated with bacterial virulence conferred by the type III secretion system (TTSS), through which P. aeruginosa causes necrosis of the lung epithelium and disseminates into the circulation, resulting in bacteremia, sepsis, and mortality. TTSS allows P. aeruginosa to directly translocate cytotoxins into eukaryotic cells, inducing cell death. The P. aeruginosa V-antigen PcrV, a homolog of the Yersinia V-antigen LcrV, is an indispensable contributor to TTS toxin translocation. Vaccination against PcrV ensures the survival of challenged mice and decreases lung inflammation and injury. Both the rabbit polyclonal anti-PcrV antibody and the murine monoclonal anti-PcrV antibody, mAb166, inhibit TTS toxin translocation. mAb166 IgG was cloned, and a molecular engineered humanized anti-PcrV IgG antigen-binding fragment, KB001, was developed for clinical use. KB001 is currently undergoing Phase-II clinical trials for ventilator-associated pneumonia in France and chronic pneumonia in cystic fibrosis in USA. In these studies, KB001 has demonstrated its safety, a favorable pharmacokinetic profile, and promising potential as a nonantibiotic strategy to reduce airway inflammation and damage in P. aeruginosa pneumonia.
Keywords: CF, cystic fibrosis; Fab, fragment antigen binding; Fc, fragment crystallizable region; MDR, multidrug resistant; MDRP, multidrug resistant Pseudomonas aeruginosa; P. aeruginosa, Pseudomonas aeruginosa; PcrV; Pseudomonas aeruginosa; TTS, type III secretory; TTSS, type III secretion system; V-antigen; VAP, ventilator-associated pneumonia; antibody; immunoglobulin G, IgG; mAb, monoclonal antibody; type III secretion system.
Figures



References
-
- Parker CM, Kutsogiannis J, Muscedere J, Cook D, Dodek P, Day AG, Heyland DK, Canadian critical care trials G. ventilator-associated pneumonia caused by multidrug-resistant organisms or Pseudomonas aeruginosa: prevalence, incidence, risk factors, and outcomes. J Crit Care 2008; 23:18-26; PMID:18359417; http://dx.doi.org/10.1016/j.jcrc.2008.02.001 - DOI - PubMed
-
- Bassetti M, Taramasso L, Giacobbe DR, Pelosi P. Management of ventilator-associated pneumonia: epidemiology, diagnosis and antimicrobial therapy. Expert Rev Anti Infect Ther 2012; 10:585-96; PMID:22702322; http://dx.doi.org/10.1586/eri.12.36 - DOI - PubMed
-
- Grgurich PE, Hudcova J, Lei Y, Sarwar A, Craven DE. Management and prevention of ventilator-associated pneumonia caused by multidrug-resistant pathogens. Expert Rev Respir Med 2012; 6:533-55; PMID:23134248; http://dx.doi.org/10.1586/ers.12.45 - DOI - PubMed
-
- Barbier F, Andremont A, Wolff M, Bouadma L. Hospital-acquired pneumonia and ventilator-associated pneumonia: recent advances in epidemiology and management. Curr Opin Pulm Med 2013; 19:216-28; PMID:23524477; http://dx.doi.org/10.1097/MCP.0b013e32835f27be - DOI - PubMed
-
- Sawa T, Wiener-Kronish JP. A therapeutic strategy against the shared virulence mechanism utilized by both Yersinia pestis and Pseudomonas aeruginosa. Anesthesiol Clin North Am 2004; 22:591-606, viii-ix; PMID:15325721 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
