Assessment of bivalent and tetravalent dengue vaccine formulations in flavivirus-naïve adults in Mexico
- PMID: 25483647
- PMCID: PMC5443102
- DOI: 10.4161/21645515.2014.972131
Assessment of bivalent and tetravalent dengue vaccine formulations in flavivirus-naïve adults in Mexico
Abstract
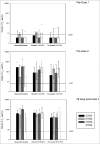
Several ChimeriVax-Dengue (CYD)-based vaccination strategies were investigated as potential alternatives to vaccination with tetravalent CYD vaccine (CYD-TDV) in this phase IIa trial conducted in 2008-9 in 150 healthy adults. Participants were randomized and vaccinated on D0 and D105 (± 15 days). One group received bivalent CYD vaccine against serotypes 1 and 3 (CYD-1;3) on day 0 and CYD-2;4 on day 105 (± 15 days). Two groups received an injection at each timepoint of a tetravalent blend of CYD-1;3;4 and a VERO cell derived, live attenuated vaccine against serotype 2 (VDV-2), or the reference CYD-TDV. A fourth group received Japanese encephalitis (JE) vaccine on days -14, -7 and 0, followed by CYD-TDV on day 105. Viraemia was infrequent in all groups. CYD-4 viraemia was most frequent after tetravalent vaccination, while CYD-3 viraemia was most frequent after the first bivalent vaccination. Immunogenicity as assessed by 50% plaque reduction neutralisation test on D28 was comparable after the first injection of either tetravalent vaccine, and increased after the second injection, particularly with the blended CYD-1;3;4/ VDV-2 vaccine. In the bivalent vaccine group, immune response against serotype 3 was highest and the second injection elicited a low immune response against CYD 2 and 4. Immune responses after the first injection of CYD-TDV in the JE-primed group were in general higher than after the first injection in the other groups. All tested regimens were well tolerated without marked differences between groups. Bivalent vaccination showed no advantage in terms of immunogenicity.
Clinical trial registration number: NCT00740155.
Keywords: ADE, antibody-dependent enhancement; AE, adverse event; ALT, aspartate aminotransferase; AST, alanine aminotransferase; CBA, cytometric bead array; CI, confidence interval; CPK, creatine phosphokinase; CYD-TDV, CYD tetravalent dengue vaccine; GMT, geometric mean titres; ICS, intracellular cytokine staining; IFN, interferon; JE, Japanese encephalitis; Japanese encephalitis; LLOQ, lower limit of quantitation; MOI, multiplicity of infection; MedDRA, medical dictionary for regulatory activities; PBMC, peripheral blood mononuclear cells; PFU, plaque forming unit; PRNT, plaque reduction neutralization test; RT-PCR, reverse transcriptase-polymerase chain reaction; TCID, tissue culture infectious dose; VDV, vero-cell adapted attenuated dengue vaccine; YF, yellow fever; dengue; flavivirus; immunogenicity; safety; vaccine.
Figures


References
-
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, et al. The global distribution and burden of dengue. Nature 2013; 496:504-7; PMID:23563266; http://dx.doi.org/ 10.1038/nature12060 - DOI - PMC - PubMed
-
- Brathwaite Dick O, San Martin JL, Montoya RH, del Diego J, Zambrano B, Dayan GH. The history of dengue outbreaks in the Americas. Am J Trop Med Hyg 2012; 87:584-93; PMID:23042846; http://dx.doi.org/ 10.4269/ajtmh.2012.11-0770 - DOI - PMC - PubMed
-
- Simmons CP, Farrar JJ, Nguyen vV, Wills B. Dengue N Engl J Med 2012; 366:1423-32; PMID:22494122; http://dx.doi.org/ 10.1056/NEJMra1110265 - DOI - PubMed
-
- Kurane I. Dengue hemorrhagic fever with special emphasis on immunopathogenesis. Comp Immunol Microbiol Infect Dis 2007; 30:329-40; PMID:17645944; http://dx.doi.org/ 10.1016/j.cimid.2007.05.010 - DOI - PubMed
-
- Murrell S, Wu SC, Butler M. Review of dengue virus and the development of a vaccine. Biotechnol Adv 2011; 29:239-47; PMID:21146601; http://dx.doi.org/ 10.1016/j.biotechadv.2010.11.008 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
