Active specific immunotherapy: using tumor heterogeneity to successfully fight cancer
- PMID: 25483649
- PMCID: PMC4514058
- DOI: 10.4161/hv.28886
Active specific immunotherapy: using tumor heterogeneity to successfully fight cancer
Keywords: Bacillus Calmette Guerin (BCG); T-cell responses; active specific immunotherapy; colon tumor; delayed-typed hypersensitivity; human monoclonal antibodies; tumor heterogeneity; tumor immunity; tumor-associated antigens.
Figures





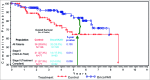
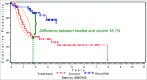
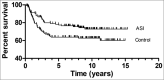


Similar articles
-
Strategies for immunotherapy of cancer.Adv Immunol. 2000;75:235-82. doi: 10.1016/s0065-2776(00)75006-1. Adv Immunol. 2000. PMID: 10879286 Review. No abstract available.
-
Tumor-specific antigens and immunologic adjuvants in cancer immunotherapy.Cancer J. 2011 Sep-Oct;17(5):325-30. doi: 10.1097/PPO.0b013e3182326004. Cancer J. 2011. PMID: 21952282 Review.
-
Human Tumor Antigens and Cancer Immunotherapy.Biomed Res Int. 2015;2015:948501. doi: 10.1155/2015/948501. Epub 2015 Jun 16. Biomed Res Int. 2015. PMID: 26161423 Free PMC article. Review.
-
Prostate cancer vaccines: current status.Semin Oncol. 1999 Apr;26(2):192-201. Semin Oncol. 1999. PMID: 10597730 Review.
-
Tumor immunology top 10 list.Immunol Rev. 2008 Apr;222:5-8. doi: 10.1111/j.1600-065X.2008.00623.x. Immunol Rev. 2008. PMID: 18363991 No abstract available.
Cited by
-
Anti-Gr-1 Antibody Provides Short-Term Depletion of MDSC in Lymphodepleted Mice with Active-Specific Melanoma Therapy.Vaccines (Basel). 2022 Apr 4;10(4):560. doi: 10.3390/vaccines10040560. Vaccines (Basel). 2022. PMID: 35455309 Free PMC article.
-
A key to the backdoor into the castle: The clinical ramifications of immunoediting driven by antigenic competition.Hum Vaccin Immunother. 2017 Jul 3;13(7):1579-1585. doi: 10.1080/21645515.2017.1301337. Epub 2017 Mar 24. Hum Vaccin Immunother. 2017. PMID: 28340323 Free PMC article. Review.
-
Therapeutic Cancer Vaccines in Colorectal Cancer: Platforms, Mechanisms, and Combinations.Cancers (Basel). 2025 Aug 6;17(15):2582. doi: 10.3390/cancers17152582. Cancers (Basel). 2025. PMID: 40805277 Free PMC article.
References
-
- Couzin-Frankel J. Breakthrough of the year 2013. cancer immunotherapy. Science 2013; 342:1432-3; PMID:24357284; http://dx.doi.org/10.1126/science.342.6165.1432 - DOI - PubMed
-
- Bucana C, Hoyer LC, Hobbs B, Breesman S, McDaniel M, Hanna MG, Jr. Morphological evidence for the translocation of lysosomal organelles from cytotoxic macrophages into the cytoplasm of tumor target cells. Cancer Res 1976; 36:4444-58; PMID:187323 - PubMed
-
- Hanna MG, Jr, Bucana C, Hobbs B, Fidler IJ. Morphologic aspects of tumor cell cytotoxicity by effector cells of the macrophage-histiocyte compartment: In vitro and in vivo studies in BCG-mediated tumor regression. In Macrophage in Neoplasia. Fink M, ed Academic Press; (1976); 113-133.
-
- Cohn ZA. The structure and function of monocytes and macrophages. Adv Immunol 1968; 9:163-214; PMID:4883741; http://dx.doi.org/10.1016/S0065-2776(08)60443-5 - DOI - PubMed
-
- Cohn ZA. Macrophage physiology. Fed Proc 1975; 34:1725-9; PMID:1093890 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
