Engineering better immunotherapies via RNA interference
- PMID: 25483669
- PMCID: PMC4514080
- DOI: 10.4161/hv.29754
Engineering better immunotherapies via RNA interference
Abstract
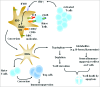
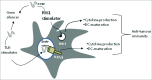
The therapeutic potential of dendritic cell (DC) cancer vaccines has gained momentum in recent years. However, clinical data indicate that antitumor immune responses generally fail to translate into measurable tumor regression. This has been ascribed to a variety of tolerance mechanisms, one of which is the expression of immunosuppressive factors by DCs and T cells. With respect to cancer immunotherapies, these factors antagonise the ability to induce robust and sustained immunity required for tumor cell eradication. Gene silencing of immunosuppressive factors in either DCs or adoptive transferred T cells enhanced anti-tumor immune responses and significantly inhibited tumor growth. Therefore, engineered next generation of DC vaccines or adoptive T-cell therapy should include immunomodulatory siRNAs to release the "brakes" imposed by the immune system. Moreover, the combination of gene silencing, antigen targeting to DCs and cytoplasmic cargo delivery will improve clinical benefits.
Keywords: AML, acute myeloid leukemia; CMV, human cytomegalovirus; CTLA4, T-lymphocyte-associated antigen 4; DC, Dendritic cells; Gal, galectin hTERT, human telomerase reverse transcriptase; IDO, indoleamine 2,3-dioxygenase; IL, interleukin; INF, interferon; NK, natural killer; PD1, programmed cell death; RNA interference; RNAi, RNA interference; SOCS1, suppressor of cytokine signaling; STAT, Signal transducer and activator of transcription; T-cell therapy; TCR, T cell receptor; TLR, toll like receptor; Treg, Regulatory T; cancer vaccine; gene silencing; immunotherapy; siRNA, small interfering RNA; targeted therapies.
Figures


Similar articles
-
Therapeutic gene modified cell based cancer vaccines.Gene. 2013 Aug 10;525(2):200-7. doi: 10.1016/j.gene.2013.03.056. Epub 2013 Apr 6. Gene. 2013. PMID: 23566846 Review.
-
Clinically feasible approaches to potentiating cancer cell-based immunotherapies.Hum Vaccin Immunother. 2015;11(4):851-69. doi: 10.1080/21645515.2015.1009814. Hum Vaccin Immunother. 2015. PMID: 25933181 Free PMC article. Review.
-
Immunosuppressive factor blockade in dendritic cells via siRNAs results in objective clinical responses.Methods Mol Biol. 2015;1218:269-76. doi: 10.1007/978-1-4939-1538-5_16. Methods Mol Biol. 2015. PMID: 25319657
-
Silencing IDO in dendritic cells: a novel approach to enhance cancer immunotherapy in a murine breast cancer model.Int J Cancer. 2013 Feb 15;132(4):967-77. doi: 10.1002/ijc.27710. Epub 2012 Jul 20. Int J Cancer. 2013. PMID: 22870862
-
Unleashing the Therapeutic Potential of Dendritic and T Cell Therapies Using RNA Interference.Methods Mol Biol. 2020;2115:259-280. doi: 10.1007/978-1-0716-0290-4_15. Methods Mol Biol. 2020. PMID: 32006406 Review.
Cited by
-
Cancer Immunotherapy: Silencing Intracellular Negative Immune Regulators of Dendritic Cells.Cancers (Basel). 2019 Jan 17;11(1):108. doi: 10.3390/cancers11010108. Cancers (Basel). 2019. PMID: 30658461 Free PMC article. Review.
-
Releasing the Immune System Brakes Using siRNAs Enhances Cancer Immunotherapy.Cancers (Basel). 2019 Feb 3;11(2):176. doi: 10.3390/cancers11020176. Cancers (Basel). 2019. PMID: 30717461 Free PMC article. Review.
-
Dendritic Cells and Programmed Death-1 Blockade: A Joint Venture to Combat Cancer.Front Immunol. 2018 Mar 1;9:394. doi: 10.3389/fimmu.2018.00394. eCollection 2018. Front Immunol. 2018. PMID: 29599770 Free PMC article. Review.
-
Cancer cell-binding peptide fused Fc domain activates immune effector cells and blocks tumor growth.Oncotarget. 2016 Nov 15;7(46):75940-75953. doi: 10.18632/oncotarget.12445. Oncotarget. 2016. PMID: 27713158 Free PMC article.
-
Recombinant Lactobacillus plantarum induces immune responses to cancer testis antigen NY-ESO-1 and maturation of dendritic cells.Hum Vaccin Immunother. 2015;11(11):2664-73. doi: 10.1080/21645515.2015.1056952. Epub 2015 Jul 17. Hum Vaccin Immunother. 2015. PMID: 26185907 Free PMC article.
References
-
- Palucka K, Banchereau J, Mellman I. Designing vaccines based on biology of human dendritic cell subsets. Immunity 2010; 33:464-78; PMID:21029958; http://dx.doi.org/10.1016/j.immuni.2010.10.007 - DOI - PMC - PubMed
-
- Liu K, Nussenzweig MC. Origin and development of dendritic cells. Immunol Rev 2010; 234:45-54. - PubMed
-
- Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol 2004; 4:336-47; PMID:15122199; http://dx.doi.org/10.1038/nri1349 - DOI - PubMed
-
- Schwartz RH. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7BB1 in interleukin-2 production and immunotherapy. Cell 1992; 71:1065-8; PMID:1335362; http://dx.doi.org/10.1016/S0092-8674(05)80055-8 - DOI - PubMed
-
- Fife BT, Pauken KE, Eagar TN, Obu T, Wu J, Tang Q, Azuma M, Krummel MF, Bluestone JA. Interaction between PD-1 and PD-L-1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol 2009; 10:1185-92; PMID:19783989; http://dx.doi.org/10.1038/ni.1790 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
