Comparative humoral and cellular immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine in healthy women aged 18-45 years: follow-up through Month 48 in a Phase III randomized study
- PMID: 25483700
- PMCID: PMC4514093
- DOI: 10.4161/hv.36117
Comparative humoral and cellular immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine in healthy women aged 18-45 years: follow-up through Month 48 in a Phase III randomized study
Abstract
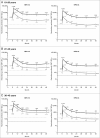
We previously reported higher anti-HPV-16 and -18 immune responses induced by HPV-16/18 vaccine compared with HPV-6/11/16/18 vaccine at Month 7 (one month after completion of full vaccination series) in women aged 18-45 y in an observer-blind study NCT00423046; the differences of immune response magnitudes were maintained up to Month 24. Here we report follow-up data through Month 48. At Month 48, in according-to-protocol cohort for immunogenicity (seronegative and DNA-negative for HPV type analyzed at baseline), geometric mean titers of serum neutralizing antibodies were 2.0- to 5.2-fold higher (HPV-16) and 8.6- to 12.8-fold higher (HPV-18) in HPV-16/18 vaccine group than in HPV-6/11/16/18 vaccine group. The majority of women in both vaccine groups remained seropositive for HPV-16. The same trend was observed for HPV-18 in HPV-16/18 vaccine group; however, seropositivity rates in HPV-6/11/16/18 vaccine group decreased considerably, particularly in the older age groups. In the total vaccinated cohort (regardless of baseline serological and HPV-DNA status), anti-HPV-16 and -18 neutralizing antibody levels induced by HPV-16/18 vaccine were higher than those induced by HPV-6/11/16/18 vaccine. CD4+ T-cell response for HPV-16 and HPV-18 was higher in HPV-16/18 vaccine group than in HPV-6/11/16/18 vaccine group. Memory B-cell responses appeared similar between vaccine groups. Both vaccines were generally well tolerated. Overall, the higher immune response observed with the HPV-16/18 vaccine was maintained up to Month 48. A head-to-head study incorporating clinical endpoints would be required to confirm whether the observed differences in immune response between the vaccines influence the duration of protection they provided.
Keywords: 50 μg) adsorbed on aluminum salt (500 μg Al(OH)3); ANOVA, analysis of variance; AS04, Adjuvant System containing 3-O-desacyl-4’-monophosphoryl lipid A (MPL; ATP, according-to-protocol; CI, confidence interval; CMI, cell-mediated immune; CVS, cervicovaginal secretion; Cervarix®; ED50, effective dose producing 50% response; ELISA, enzyme-linked immunosorbent assay; GM, geometric mean; GMR, geometric mean (titer) ratio; GMT, geometric mean titer; Gardasil®; HPA, Health Protection Agency; HPV, human papillomavirus; IgG, immunoglobulin G; MSC, medically significant condition; NOAD, new onset autoimmune disease; NOCD, new onset chronic disease; PBMC, peripheral blood mononuclear cells; PBNA, pseudovirion-based neutralization assay; SAE, serious adverse event; TVC, total vaccinated cohort; VLP, virus-like particle; human papillomavirus; immunogenicity; nAb(s), neutralizing antibody(ies); safety.
Figures

References
-
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Munoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12-9; PMID:10451482; http://dx.doi.org/10.1002/(SICI)1096-9896(199909)189:1%3c12::AID-PATH431... - DOI - PubMed
-
- Winer RL, Lee S-K, Hughes JP, Adam DE, Kiviat NB, Koutsky LA. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol 2003; 157:218-26; PMID:12543621; http://dx.doi.org/10.1002/(SICI)1096-9896(199909)189:1%3c12::AID-PATH431... - DOI - PubMed
-
- Partridge JM, Hughes JP, Feng Q, Winer RL, Weaver BA, Xi L-F, Stern ME, Lee S-K, O'Reilly SF, Hawes SE, et al. . Genital human papillomavirus infection in men: incidence and risk factors in a cohort of university students. J Infect Dis 2007; 196:1125-36; PMID:17955429; http://dx.doi.org/10.1002/(SICI)1096-9896(199909)189:1%3c12::AID-PATH431... - DOI - PubMed
-
- Castellsague X, Schneider A, Kaufmann AM, Bosch FX. HPV vaccination against cervical cancer in women above 25 years of age: key considerations and current perspectives. Gynecol Oncol 2009; 115:S15-23; PMID:19819540; http://dx.doi.org/10.1002/(SICI)1096-9896(199909)189:1%3c12::AID-PATH431... - DOI - PubMed
-
- WHO/ICO Information Centre on HPV and Cervical Cancer. HPV and cervical cancer in the 2007 report. Vaccine 2007; 25 Suppl 3:C1-26; http://dx.doi.org/10.1002/(SICI)1096-9896(199909)189:1%3c12::AID-PATH431... - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
