A general strategy for generating intact, full-length IgG antibodies that penetrate into the cytosol of living cells
- PMID: 25484049
- PMCID: PMC4622575
- DOI: 10.4161/mabs.36389
A general strategy for generating intact, full-length IgG antibodies that penetrate into the cytosol of living cells
Abstract
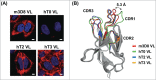
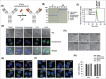
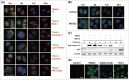
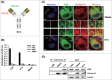
Full-length IgG antibodies cannot cross cell membranes of living cells; this limits their use for direct targeting of cytosolic proteins. Here, we describe a general strategy for the generation of intact, full-length IgG antibodies, herein called cytotransmabs, which internalize into living cells and localize in the cytosol. We first generated a humanized light chain variable domain (VL) that could penetrate into the cytosol of living cells and was engineered for association with various subtypes of human heavy chain variable domains (VHs). When light chains with humanized VL were co-expressed with 3 heavy chains (HCs), including 2 HCs of the clinically approved adalimumab (Humira®) and bevacizumab (Avastin®), all 3 purified IgG antibodies were internalized into the cytoplasm of living cells. Cytotransmabs primarily internalized into living cells by the clathrin-mediated endocytic pathway through interactions with heparin sulfate proteoglycan that was expressed on the cell surface. The cytotransmabs escaped into the cytosol from early endosomes without being further transported into other cellular compartments, like the lysosomes, endoplasmic reticulum, Golgi apparatus, and nucleus. Furthermore, we generated a cytotransmab that co-localized with the targeted cytosolic protein when it was incubated with living cells, demonstrating that the cytotransmab can directly target cytosolic proteins. Internalized cytotransmabs did not show any noticeable cytotoxicity and remained in the cytosol for more than 6 h before being degraded by proteosomes. These results suggest that cytotransmabs, which efficiently enter living cells and reach the cytosolic space, will find widespread uses as research, diagnostic, and therapeutic agents.
Keywords: CDR, complementarity-determining region; HC, heavy chain; IgG antibody; IgG, immunoglobulin G; LC, light chain; VH, heavy chain variable domain; VL, light chain variable domain; cell-penetrating antibody; cellular internalization; cytosolic protein targeting; endosomal release; intracellular trafficking; next-generation antibody.
Figures

References
-
- Marschall AL, Frenzel A, Schirrmann T, Schungel M, Dubel S. Targeting antibodies to the cytoplasm. MAbs 2011; 3:3-16; PMID:21099369; http://dx.doi.org/10.4161/mabs.3.1.14110 - DOI - PMC - PubMed
-
- Ivanov AA, Khuri FR, Fu H. Targeting protein-protein interactions as an anticancer strategy. Trends Pharmacol Sci 2013; 34:393-400; PMID:23725674; http://dx.doi.org/10.1016/j.tips.2013.04.007 - DOI - PMC - PubMed
-
- Freund G, Sibler AP, Desplancq D, Oulad-Abdelghani M, Vigneron M, Gannon J, Van Regenmortel MH, Weiss E. Targeting endogenous nuclear antigens by electrotransfer of monoclonal antibodies in living cells. MAbs 2013; 5:518-22; PMID:23765067; http://dx.doi.org/10.4161/mabs.25084 - DOI - PMC - PubMed
-
- Weill CO, Biri S, Adib A, Erbacher P. A practical approach for intracellular protein delivery. Cytotechnology 2008; 56:41-8; PMID:19002840; http://dx.doi.org/10.1007/s10616-007-9102-3 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
