V-ATPase and osmotic imbalances activate endolysosomal LC3 lipidation
- PMID: 25484071
- PMCID: PMC4502810
- DOI: 10.4161/15548627.2014.984277
V-ATPase and osmotic imbalances activate endolysosomal LC3 lipidation
Abstract
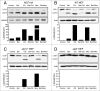
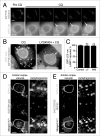
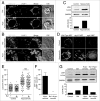
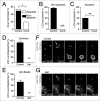
Recently a noncanonical activity of autophagy proteins has been discovered that targets lipidation of microtubule-associated protein 1 light chain 3 (LC3) onto macroendocytic vacuoles, including macropinosomes, phagosomes, and entotic vacuoles. While this pathway is distinct from canonical autophagy, the mechanism of how these nonautophagic membranes are targeted for LC3 lipidation remains unclear. Here we present evidence that this pathway requires activity of the vacuolar-type H(+)-ATPase (V-ATPase) and is induced by osmotic imbalances within endolysosomal compartments. LC3 lipidation by this mechanism is induced by treatment of cells with the lysosomotropic agent chloroquine, and through exposure to the Heliobacter pylori pore-forming toxin VacA. These data add novel mechanistic insights into the regulation of noncanonical LC3 lipidation and its associated processes, including LC3-associated phagocytosis (LAP), and demonstrate that the widely and therapeutically used drug chloroquine, which is conventionally used to inhibit autophagy flux, is an inducer of LC3 lipidation.
Keywords: ATG, autophagy-related; Baf, bafilomycin A1; CALCOCO2/NDP52, calcium binding and coiled-coil domain 2; CQ, chloroquine; ConA, concanamycin A; FYCO1, FYVE and coiled-coil domain containing 1; GFP, green fluorescent protein; Helicobacter pylori; LAMP1, lysosomal-associated membrane protein 1; LAP; LAP, LC3-associated phagocytosis; LC3; MAP1LC3/LC3, microtubule-associated protein 1 light chain 3; MTOR, mechanistic target of rapamycin; PIK3C3/VPS34, phosphatidylinositol 3-kinase; PtdIns3K, phosphatidylinositol 3-kinase; PtdIns3P, phosphatidylinositol 3-phosphate; RB1CC1/FIP200, RB1-inducible coiled-coil 1; SQSTM1/p62, sequestosome 1; TEM, transmission electron microscopy; TLR, toll-like receptor; ULK1/2, unc-51 like autophagy activating kinase 1/2; V-ATPase; V-ATPase, vacuolar-type H+-ATPase; VacA, vacuolating toxin A; autophagy; catalytic subunit type 3; chloroquine; entosis; lysosome; phagocytosis.
Figures


References
-
- Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. New Engl J Med 2013; 368:651-62; PMID:23406030; http://dx.doi.org/ 10.1056/NEJMra1205406 - DOI - PubMed
-
- Feng Y, He D, Yao Z, Klionsky DJ. The machinery of macroautophagy. Cell Res 2014; 24:24-41; PMID:24366339; http://dx.doi.org/ 10.1038/cr.2013.16 - DOI - PMC - PubMed
-
- Florey O, Overholtzer M. Autophagy proteins in macroendocytic engulfment. Trends Cell Biol 2012; 22:374-80; PMID:22608991; http://dx.doi.org/ 10.1016/j.tcb.2012.04.005 - DOI - PMC - PubMed
-
- Sanjuan MA, Dillon CP, Tait SW, Moshiach S, Dorsey F, Connell S, Komatsu M, Tanaka K, Cleveland JL, Withoff S, et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature 2007; 450:1253-7; PMID:18097414; http://dx.doi.org/ 10.1038/nature06421 - DOI - PubMed
-
- Kim JY, Zhao H, Martinez J, Doggett TA, Kolesnikov AV, Tang PH, Ablonczy Z, Chan CC, Zhou Z, Green DR, et al. Noncanonical autophagy promotes the visual cycle. Cell 2013; 154:365-76; PMID:23870125; http://dx.doi.org/ 10.1016/j.cell.2013.06.012 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
