Expression, purification and X-ray crystallographic analysis of the Helicobacter pylori blood group antigen-binding adhesin BabA
- PMID: 25484214
- PMCID: PMC4259228
- DOI: 10.1107/S2053230X14023188
Expression, purification and X-ray crystallographic analysis of the Helicobacter pylori blood group antigen-binding adhesin BabA
Abstract
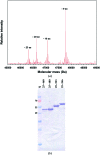
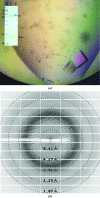
Helicobacter pylori is a human pathogen that colonizes about 50% of the world's population, causing chronic gastritis, duodenal ulcers and even gastric cancer. A steady emergence of multiple antibiotic resistant strains poses an important public health threat and there is an urgent requirement for alternative therapeutics. The blood group antigen-binding adhesin BabA mediates the intimate attachment to the host mucosa and forms a major candidate for novel vaccine and drug development. Here, the recombinant expression and crystallization of a soluble BabA truncation (BabA(25-460)) corresponding to the predicted extracellular adhesin domain of the protein are reported. X-ray diffraction data for nanobody-stabilized BabA(25-460) were collected to 2.25 Å resolution from a crystal that belonged to space group P21, with unit-cell parameters a = 50.96, b = 131.41, c = 123.40 Å, α = 90.0, β = 94.8, γ = 90.0°, and which was predicted to contain two BabA(25-460)-nanobody complexes per asymmetric unit.
Keywords: BabA; Helicobacter pylori; adhesin; nanobody.
Figures

Similar articles
-
The three-dimensional structure of the extracellular adhesion domain of the sialic acid-binding adhesin SabA from Helicobacter pylori.J Biol Chem. 2014 Mar 7;289(10):6332-6340. doi: 10.1074/jbc.M113.513135. Epub 2013 Dec 27. J Biol Chem. 2014. PMID: 24375407 Free PMC article.
-
Dynamics of Lewis b binding and sequence variation of the babA adhesin gene during chronic Helicobacter pylori infection in humans.mBio. 2014 Dec 16;5(6):e02281-14. doi: 10.1128/mBio.02281-14. mBio. 2014. PMID: 25516619 Free PMC article.
-
[Screening and obataining of aptamers for the blood group antigen-binding adhesin (BabA) to block Helicobacter pylori (H.pylori) colonization in the stomach of mice].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2023 Sep;39(9):793-800. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2023. PMID: 37732574 Chinese.
-
Helicobacter pylori adhesins: HpaA a potential antigen in experimental vaccines for H. pylori.Helicobacter. 2021 Feb;26(1):e12758. doi: 10.1111/hel.12758. Epub 2020 Dec 1. Helicobacter. 2021. PMID: 33259676 Review.
-
Helicobacter pylori BabA-SabA Key Roles in the Adherence Phase: The Synergic Mechanism for Successful Colonization and Disease Development.Toxins (Basel). 2021 Jul 13;13(7):485. doi: 10.3390/toxins13070485. Toxins (Basel). 2021. PMID: 34357957 Free PMC article. Review.
Cited by
-
Structural Insights into Polymorphic ABO Glycan Binding by Helicobacter pylori.Cell Host Microbe. 2016 Jan 13;19(1):55-66. doi: 10.1016/j.chom.2015.12.004. Cell Host Microbe. 2016. PMID: 26764597 Free PMC article.
-
Structural basis of Lewis(b) antigen binding by the Helicobacter pylori adhesin BabA.Sci Adv. 2015 Aug 14;1(7):e1500315. doi: 10.1126/sciadv.1500315. eCollection 2015 Aug. Sci Adv. 2015. PMID: 26601230 Free PMC article.
-
Host Determinants of Expression of the Helicobacter pylori BabA Adhesin.Sci Rep. 2017 Apr 18;7:46499. doi: 10.1038/srep46499. Sci Rep. 2017. PMID: 28418004 Free PMC article.
-
Ferric quinate (QPLEX) inhibits the interaction of major outer membrane protein (MOMP) with the Lewis b (Leb) antigen and limits Campylobacter colonization in broilers.Front Microbiol. 2023 Mar 10;14:1146418. doi: 10.3389/fmicb.2023.1146418. eCollection 2023. Front Microbiol. 2023. PMID: 36970690 Free PMC article.
-
Ferric quinate (QPLEX) interacts with the major outer membrane protein (MOMP) of Campylobacter jejuni and enters through the porin channel into the periplasmic space.Comput Struct Biotechnol J. 2022 Sep 24;20:5355-5363. doi: 10.1016/j.csbj.2022.09.032. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 36212543 Free PMC article.
References
-
- Aspholm-Hurtig, M. et al. (2004). Science, 305, 519–522. - PubMed
-
- Backert, S. & Clyne, M. (2011). Helicobacter, 16, 19–25. - PubMed
-
- Baranova, E., Fronzes, R., Garcia-Pino, A., Van Gerven, N., Papapostolou, D., Péhau-Arnaudet, G., Pardon, E., Steyaert, J., Howorka, S. & Remaut, H. (2013). Nature (London), 487, 119–122. - PubMed
-
- Borén, T., Falk, P., Roth, K. A., Larson, G. & Normark, S. (1993). Science, 262, 1892–1895. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

