Optimization of the crystallizability of a single-chain antibody fragment
- PMID: 25484230
- PMCID: PMC4259244
- DOI: 10.1107/S2053230X1402247X
Optimization of the crystallizability of a single-chain antibody fragment
Abstract
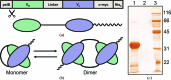
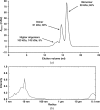
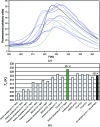
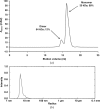
Single-chain variable antibody fragments (scFvs) are molecules with immense therapeutic and diagnostic potential. Knowledge of their three-dimensional structure is important for understanding their antigen-binding mode as well as for protein-engineering approaches such as antibody humanization. A major obstacle to the crystallization of single-chain variable antibody fragments is their relatively poor homogeneity caused by spontaneous oligomerization. A new approach to optimization of the crystallizability of single-chain variable antibody fragments is demonstrated using a representative single-chain variable fragment derived from the anti-CD3 antibody MEM-57. A Thermofluor-based assay was utilized to screen for optimal conditions for antibody-fragment stability and homogeneity. Such an optimization of the protein storage buffer led to a significantly improved ability of the scFv MEM-57 to yield crystals.
Keywords: Thermofluor assay; crystallizability optimization; crystallization; differential scanning fluorimetry; oligomerization; single-chain antibody fragment.
Figures

Similar articles
-
High-level secretion of recombinant monomeric murine and human single-chain Fv antibodies from Drosophila S2 cells.Protein Eng Des Sel. 2012 Feb;25(2):59-66. doi: 10.1093/protein/gzr058. Epub 2011 Dec 12. Protein Eng Des Sel. 2012. PMID: 22160929 Free PMC article.
-
Conversion of scFv peptide-binding specificity for crystal chaperone development.Protein Eng Des Sel. 2011 May;24(5):419-28. doi: 10.1093/protein/gzq120. Epub 2011 Jan 8. Protein Eng Des Sel. 2011. PMID: 21217145 Free PMC article.
-
Comprehensive optimization of a single-chain variable domain antibody fragment as a targeting ligand for a cytotoxic nanoparticle.MAbs. 2015;7(1):42-52. doi: 10.4161/19420862.2014.985933. MAbs. 2015. PMID: 25484041 Free PMC article.
-
ScFv Improvement Approaches.Protein Pept Lett. 2018;25(3):222-229. doi: 10.2174/0929866525666171129225436. Protein Pept Lett. 2018. PMID: 29189116 Review.
-
[Single chain antibody fragment display systems: a review].Sheng Wu Gong Cheng Xue Bao. 2023 Sep 25;39(9):3681-3694. doi: 10.13345/j.cjb.220911. Sheng Wu Gong Cheng Xue Bao. 2023. PMID: 37805846 Review. Chinese.
References
-
- Arndt, K. M., Müller, K. M. & Plückthun, A. (1998). Biochemistry, 37, 12918–12926. - PubMed
-
- Atwell, J. L., Breheney, K. A., Lawrence, L. J., McCoy, A. J., Kortt, A. A. & Hudson, P. J. (1999). Protein Eng. 12, 597–604. - PubMed
-
- Bayly, A. M., Kortt, A. A., Hudson, P. J. & Power, B. E. (2002). J. Immunol. Methods, 262, 217–227. - PubMed
-
- Bird, R. E., Hardman, K. D., Jacobson, J. W., Johnson, S., Kaufman, B. M., Lee, S. M., Lee, T., Pope, S. H., Riordan, G. S. & Whitlow, M. (1988). Science, 242, 423–426. - PubMed
-
- Carmichael, J. A., Power, B. E., Garrett, T. P. J., Yazaki, P. J., Shively, J. E., Raubischek, A. A., Wu, A. M. & Hudson, P. J. (2003). J. Mol. Biol. 326, 341–351. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

