Aurora kinases phosphorylate Lgl to induce mitotic spindle orientation in Drosophila epithelia
- PMID: 25484300
- PMCID: PMC4291145
- DOI: 10.1016/j.cub.2014.10.052
Aurora kinases phosphorylate Lgl to induce mitotic spindle orientation in Drosophila epithelia
Abstract
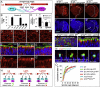
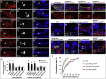
The Lethal giant larvae (Lgl) protein was discovered in Drosophila as a tumor suppressor in both neural stem cells (neuroblasts) and epithelia. In neuroblasts, Lgl relocalizes to the cytoplasm at mitosis, an event attributed to phosphorylation by mitotically activated aPKC kinase and thought to promote asymmetric cell division. Here we show that Lgl also relocalizes to the cytoplasm at mitosis in epithelial cells, which divide symmetrically. The Aurora A and B kinases directly phosphorylate Lgl to promote its mitotic relocalization, whereas aPKC kinase activity is required only for polarization of Lgl. A form of Lgl that is a substrate for aPKC, but not Aurora kinases, can restore cell polarity in lgl mutants but reveals defects in mitotic spindle orientation in epithelia. We propose that removal of Lgl from the plasma membrane at mitosis allows Pins/LGN to bind Dlg and thus orient the spindle in the plane of the epithelium. Our findings suggest a revised model for Lgl regulation and function in both symmetric and asymmetric cell divisions.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Bryant P.J., Schubiger G. Giant and duplicated imaginal discs in a new lethal mutant of Drosophila melanogaster. Dev. Biol. 1971;24:233–263. - PubMed
-
- Jacob L., Opper M., Metzroth B., Phannavong B., Mechler B.M. Structure of the l(2)gl gene of Drosophila and delimitation of its tumor suppressor domain. Cell. 1987;50:215–225. - PubMed
-
- Bilder D., Li M., Perrimon N. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science. 2000;289:113–116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

