Nrf2 regulates ROS production by mitochondria and NADPH oxidase
- PMID: 25484314
- PMCID: PMC4471129
- DOI: 10.1016/j.bbagen.2014.11.021
Nrf2 regulates ROS production by mitochondria and NADPH oxidase
Abstract
Background: Nuclear factor (erythroid-derived 2) factor 2 (Nrf2) is a crucial transcription factor mediating protection against oxidants. Nrf2 is negatively regulated by cytoplasmic Kelch-like ECH associated protein 1 (Keap1) thereby providing inducible antioxidant defence. Antioxidant properties of Nrf2 are thought to be mainly exerted by stimulating transcription of antioxidant proteins, whereas its effects on ROS production within the cell are uncertain.
Methods: Live cell imaging and qPCR in brain hippocampal glio-neuronal cultures and explants slice cultures with graded expression of Nrf2, i.e. Nrf2-knockout (Nrf2-KO), wild-type (WT), and Keap1-knockdown (Keap1-KD).
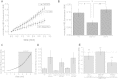
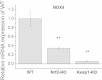
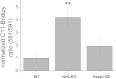
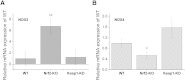
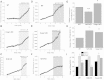
Results: We here show that ROS production in Nrf2-KO cells and tissues is increased compared to their WT counterparts. Mitochondrial ROS production is regulated by the Keap1-Nrf2 pathway by controlling mitochondrial bioenergetics. Surprisingly, Keap1-KD cells and tissues also showed higher rates of ROS production when compared to WT, although with a smaller magnitude. Analysis of the mRNA expression levels of the two NOX isoforms implicated in brain pathology showed, that NOX2 is dramatically upregulated under conditions of Nrf2 deficiency, whereas NOX4 is upregulated when Nrf2 is constitutively activated (Keap1-KD) to a degree which paralleled the increases in ROS production.
Conclusions: These observations suggest that the Keap1-Nrf2 pathway regulates both mitochondrial and cytosolic ROS production through NADPH oxidase.
General significance: Findings supports a key role of the Keap1-Nrf2 pathway in redox homeostasis within the cell.
Keywords: Keap1; NADPH oxidase; NOX; Nrf2; ROS.
Copyright © 2014. Published by Elsevier B.V.
Figures

References
-
- Baird L., Dinkova-Kostova A.T. The cytoprotective role of the Keap1–Nrf2 pathway. Arch. Toxicol. 2011;85:241–272. - PubMed
-
- Motohashi H., Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004;10:549–557. - PubMed
-
- Domijan A.-M., Kovac S., Abramov A.Y. Lipid peroxidation is essential for phospholipase C activity and the inositol-trisphosphate-related Ca2 + signal. J. Cell Sci. 2014;127:21–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

