The Development of Visible-Light Photoredox Catalysis in Flow
- PMID: 25484447
- PMCID: PMC4255365
- DOI: 10.1002/ijch.201300136
The Development of Visible-Light Photoredox Catalysis in Flow
Abstract
Visible-light photoredox catalysis has recently emerged as a viable alternative for radical reactions otherwise carried out with tin and boron reagents. It has been recognized that by merging photoredox catalysis with flow chemistry, slow reaction times, lower yields, and safety concerns may be obviated. While flow reactors have been successfully applied to reactions carried out with UV light, only recent developments have demonstrated the same potential of flow reactors for the improvement of visible-light-mediated reactions. This review examines the initial and continuing development of visible-light-mediated photoredox flow chemistry by exemplifying the benefits of flow chemistry compared with conventional batch techniques.
Keywords: Photochemistry; Radical reactions; Radicals; Redox chemistry.
Figures
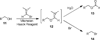


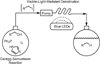












References
-
- Wiles C, Watts P. Eur. J. Org. Chem. 2008;10:1655–1671.
-
- Trommsdorff H. Ann. Chem. Pharm. 1834;11:190–208.
-
- Ninomiya I, Naito T, editors. Best Synthetic Methods: Photochemical Synthesis. London: Academic Press; 1989.
-
- Hook BDA, Dohle W, Hirst PR, Pickworth M, Berry MB, Booker-Milburn KI. J. Org. Chem. 2005;70:7558–7564. - PubMed
- Goodell JR, McMullen JP, Zaborenko N, Maloney JR, Ho KF, Jensen C-H, Porco JA, Beeler AB. J. Org. Chem. 2009;74:6169–6180. - PMC - PubMed
- Bou-Hamdan FR, Seeberger PH. Chem. Sci. 2012;3:1612–1616.
- Tucker JW, Zhang Y, Jamison TF, Stephenson CRJ. Angew. Chem. Int. Ed. 2012;51:4144–4147. - PMC - PubMed
- Andrews RS, Becker JJ, Gagné MR. Angew. Chem. Int. Ed. 2012;51:4140–4143. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
