Palliative treatment of superior vena cava syndrome with nitinol stents
- PMID: 25484557
- PMCID: PMC4244246
- DOI: 10.1055/s-0034-1383432
Palliative treatment of superior vena cava syndrome with nitinol stents
Abstract
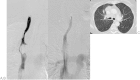
This study aims to retrospectively evaluate the outcomes following nitinol stent placement for malignant superior vena cava syndrome. A total of 25 patients with thoracic malignancies were treated with self-expanding nitinol stents for superior vena cava syndrome (E*Luminexx [Bard GmbH/Angiomed, Karlsruhe, Germany], Sinus-XL [OptiMed Medizinische Instrumente GmbH, Ettlingen, Germany], and Zilver Vena [Cook Medical Inc., Bloomington, IN]). It was seen that the procedural success rate was 76% with all stents deployed as intended and no procedure-related complications but in five patients with 50% residual stenosis and one patient with stent occlusion within 48 hours after stent deployment. Stent occlusion occurred in further two patients during follow-up: one patient developed infection, thrombosis, and occlusion in the stent seen at 2-month follow-up, and one patient had stent occlusion at 4-month follow-up. The clinical success rate was 96%. Stent compression leading to a greater than 50% reduction in stent diameter was observed in three patients at follow-up. Overall 22 patients died at a mean follow-up of 3.5 months for reasons related to their underlying malignancy. It was concluded that the stent treatment for superior vena cava syndrome is a safe treatment with good clinical effect in patients with superior vena cava syndrome in the terminal phase of malignant disease. In this small patient population, no trends were observed which would suggest that outcomes vary by stent type, though additional, large-scale studies are needed.
Keywords: interventional radiology; palliative; stent; superior vena cava syndrome; treatment.
Conflict of interest statement
Figures




Similar articles
-
A new nitinol stent for use in superior vena cava syndrome. Initial clinical experience.J Cardiovasc Surg (Torino). 2015 Dec;56(6):877-81. Epub 2015 Jul 27. J Cardiovasc Surg (Torino). 2015. PMID: 26212865
-
Retrospective study in 23 patients of the self-expanding sinus-XL stent for treatment of malignant superior vena cava obstruction caused by non-small cell lung cancer.J Vasc Interv Radiol. 2015 Mar;26(3):357-65. doi: 10.1016/j.jvir.2014.11.019. Epub 2015 Jan 28. J Vasc Interv Radiol. 2015. PMID: 25638748
-
Results of Palliative Stenting in Malignant Superior Vena Cava Syndrome Analyzing Self-Expanding Stainless Steel and Nitinol Venous Bare Metal Stents.J Endovasc Ther. 2024 Apr 27:15266028241242926. doi: 10.1177/15266028241242926. Online ahead of print. J Endovasc Ther. 2024. PMID: 38676408
-
Endovascular stenting for end-stage lung cancer patients with superior vena cava syndrome post first-line treatments - A single-center experience and literature review.J Chin Med Assoc. 2017 Aug;80(8):482-486. doi: 10.1016/j.jcma.2017.04.005. Epub 2017 May 10. J Chin Med Assoc. 2017. PMID: 28501315 Review.
-
Safety and effectiveness of vascular endoprosthesis for malignant superior vena cava syndrome.Thorax. 2009 Feb;64(2):174-8. doi: 10.1136/thx.2007.086017. Thorax. 2009. PMID: 19176843 Review.
Cited by
-
Vascular spinal cord obstruction associated with superior vena cava syndrome: A case report and literature review.Medicine (Baltimore). 2017 Dec;96(51):e9196. doi: 10.1097/MD.0000000000009196. Medicine (Baltimore). 2017. PMID: 29390464 Free PMC article. Review.
-
Percutaneous transluminal stenting for superior vena cava syndrome caused by malignant tumors: a single-center retrospective study.J Cardiothorac Surg. 2021 Mar 20;16(1):39. doi: 10.1186/s13019-021-01418-w. J Cardiothorac Surg. 2021. PMID: 33743767 Free PMC article.
-
Superior Vena Cava Syndrome and Wallstent: A Systematic Review.Ann Vasc Dis. 2022 Jun 25;15(2):87-93. doi: 10.3400/avd.ra.21-00118. Ann Vasc Dis. 2022. PMID: 35860826 Free PMC article.
-
Malignant obstruction of the inferior vena cava: clinical experience with the self-expanding Sinus-XL stent system.Abdom Radiol (NY). 2022 Oct;47(10):3604-3614. doi: 10.1007/s00261-022-03587-1. Epub 2022 Jul 6. Abdom Radiol (NY). 2022. PMID: 35790568 Free PMC article.
-
Endovascular therapy for superior vena cava syndrome: A systematic review and meta-analysis.EClinicalMedicine. 2021 Jun 28;37:100970. doi: 10.1016/j.eclinm.2021.100970. eCollection 2021 Jul. EClinicalMedicine. 2021. PMID: 34386747 Free PMC article.
References
-
- Uberoi R. Quality assurance guidelines for superior vena cava stenting in malignant disease. Cardiovasc Intervent Radiol. 2006;29(3):319–322. - PubMed
-
- Kee S T, Kinoshita L, Razavi M K, Nyman U R, Semba C P, Dake M D. Superior vena cava syndrome: treatment with catheter-directed thrombolysis and endovascular stent placement. Radiology. 1998;206(1):187–193. - PubMed
-
- Wan J F, Bezjak A. Superior vena cava syndrome. Hematol Oncol Clin North Am. 2010;24(3):501–513. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

